The equilibrium constant at 25 C is 1.58 10 2 for Calculate G and E for
Question:
The equilibrium constant at 25 °C is 1.58 × 102 for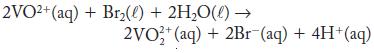
Calculate ΔG° and E° for this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
It looks like you have provided an image of a chemical reaction with a given equilibrium constant K ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The budgeted income statement for Barnaby's Hideaway is produced on your Excel spreadsheet. Assume that the following constitute the fixed and vari- able costs for the upcoming year Fixed Costs for...
-
As in Example 6L.1, you are planning to use a Daniell cell to power a model electric car. However, you find that you do not have standard solutions available. You have only dilute solutions, and you...
-
Case1. 640,000 viewers interested in Sports and 360,000 viewers interested in Economy C2 Sports Economy V3 Sports 32,32 36,64 Economy 64, 36 18,18 Questions: 1. What is the equilibrium of the game?...
-
The Regina Company, Inc. BALANCE SHEET (In Thousands) ASSETS Cash Accounts Receivable Inventories Other Current Assets Total Current Assets Fixed Assets Accum Depreciation Other Assets TOTAL ASSETS...
-
A long, cylindrical heating element of 20-mm diameter operating at 700 K in vacuum is located 40 mm from an insulated wall of low thermal conductivity (a) Assuming both the element and the wall are...
-
Ethernet and wireless networks have some similarities and some differences. One property of Ethernet is that only one frame at a time can be transmitted on an Ethernet. Do 802.11 share this property...
-
A random variable \(U\) is uniformly distributed on the interval \((-A, A)\) and its probability density function is zero otherwise. (a) Find an expression for \(A\) in terms of the variance...
-
The following events were experienced by Abbot Inc.: 1. Issued cumulative preferred stock for cash. 2. Issued common stock for cash. 3. Distributed a 2-for-1 stock split on the common stock . 4....
-
A roller coaster car (RC) of mass m = 1200 kg (includes passengers) is to be thrust into motion from a height H with a speed of vo = 2 m/s. It is to come down the track and complete a full circle...
-
Calculate the potential of the half-reaction when the concentrations in solution are [Fe 3+ ] = 0.033 M and [Fe 2+ ] = 0.0025 M, and the temperature is 298 K.
-
The standard free energy change at 25 C, G, is equal to -34.3 kJ for Calculate the standard potential for this reaction.
-
A rain gutter is made from sheets of aluminum that are 12 inches wide by turning up the edges to form right angles. Determine the depth of the gutter that will maximize its cross-sectional area and...
-
On what grounds can an insurance contract be terminated? a. What has complicated voluntary termination? b. What steps must an insurer take to terminate an insureds policy?
-
________ has allowed recovery by plaintiffs who would have been excluded by contributory negligence.
-
Why have some states limited or eliminated the collateral-source rule?
-
True Or False Progressive Era reformers advocated the adoption of joint and several liability because they thought it was important to protect negligent tortfeasors from disproportionate liability.
-
True Or False Classical reformers disliked joint and several liability because they believed that plaintiffs should bear the risk of insolvent multiple defendants just as they did when there was only...
-
Algonquin, Inc., reported the following items at December 31, 2012, and 2011: Requirements 1. Compute Algonquins (a) quick (acid-test) ratio and (b) days sales in average receivables for 2012....
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Beginning with Eq. (4.38), show that for two dielectric media, in general tan θ p = [ϵ t (ϵ t μ i - ϵ i μ t...
-
Show that the polarization angles for internal and external reflection at a given interface are complementary, that is, p + ' p = 90 (see Problem 4.66). Data from Prob. 4.66 Show that tan p = n t...
-
It is often useful to work with the azimuthal angle γ, which is defined as the angle between the plane-of-vibration and the plane-of-incidence. Thus for linearly polarized light, Figure...
-
It is very important to have a clear understanding of the responsibilities of a bookkeeper, while also being aware of your own capabilities and limitations. This will enable you to identify when...
-
July 31st Revenue 7900 Retained Earnings 7900 July 31st Income Summary 5200 Rent Expense 400 Wages Expense 1750 Repairs & Maintenance Expense 350 Supplies Expense 1400 Depriciation Expense 100 Income...
-
Good Slings Inc. manufactures several wood and string instruments. In particular, it is well-known for its production of classic violins. Assume that Good Slings uses a periodic inventory system and...

Study smarter with the SolutionInn App


