The most intense peak in a mass spectrum is assigned a height of 100 units. The following
Question:
The most intense peak in a mass spectrum is assigned a height of 100 units. The following spectrum was obtained from a sample of an element. Use the data to calculate the atomic mass of the element. Identify the element.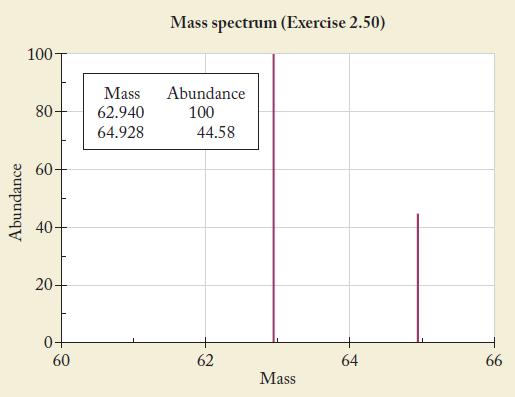
Transcribed Image Text:
Abundance 100- 80- 60 40 20- 60 Mass 62.940 64.928 Mass spectrum (Exercise 2.50) Abundance 100 44.58 62 Mass 64 66
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To calculate the average atomic mass of the element from the mass spectrum you need to consider both ...View the full answer

Answered By

Shivaji Thombare
I am Mr. Shivaji Thombare.
*Qualification*
M.Sc. Mathematics
(Savitribai Phule Pune University, Pune,India
B.Ed(Bachelor's degree in education)
(Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.)
*Experience*
I have worked as a Lecturer in Mathematics for 6 years in Jamkhed Mahavidyalaya,Jamkhed,India.I also tutor at Chegg and helped students all over world to solve mathematics problems for 2 Years. I am an easy-going person and see Mathematics as fun. I would like to help students in Mathematics. I love to teach students and see them succeed in life.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The most intense peak in a mass spectrum is assigned a height of 100 units. The following spectrum was obtained from a sample of an element. Use the data to calculate the atomic mass of the element....
-
The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic of Pb. The most intense peak in each envelope appears at m/z 326.0, 267.0, 224.0 and...
-
Polystyrene is a synthetic polymer with the structure - (CH2- CH (C6Hs)), A batch of polydisperse polystyrene was prepared by initiating the polymerization with t-butyl radicals. As a result, the...
-
The following is the stockholders' equity section from Chipotle Mexican Grill, Inc.'s balance sheet (in thousands, except per share data). a. Show the computation to derive the $375 thousand for...
-
A waveguide with lossless dielectric inside and perfectly conducting walls has a cross-sectional contour ? that departs slightly from a comparison contour Co whose fields are known. The difference in...
-
Explain how New Pigs marketing communication helps support and build the brand image. In what ways do the other elements of the marketing mix communicate messages about the New Pig brand?
-
Is the coefficient of restitution \(e\) different in two inertial reference frames, which are moving at constant velocity relative to each other? (See Eq. 5.18 if you have forgotten the definition of...
-
Hopkins Community Hospital operates an outpatient clinic in a town several miles from the main hospital. For several years the clinic has struggled just to break even. The clinics financial budget...
-
7. 8. The work done by a gas molecule in an isolated system is given by W=ae KT, where x is the displacement, k is the Boltzmann constant and T is the temperature. and are constants. Then the...
-
Antimony occurs naturally as two isotopes, one with a mass of 120.904 u and the other with a mass of 122.904 u. (a) Give the symbol that identifies each of these isotopes of antimony. (b) Get the...
-
The mass spectrum of an element shows that 92.2% of the atoms have a mass of 27.977 u, 4.67% have a mass of 28.976 u, and the remaining 3.10% have a mass of 29.974 u. (a) Calculate the atomic mass...
-
If f(x) = xe sin x, find f(x) graph f and f(x) f on the same screen and comment
-
What is the role of codetermination? In your opinion, is the incorporation of lower-level employees on the board appropriate?
-
Provide local examples to show the relevance of Hofstedes dimensions in effective staffing and directing.
-
How does VRIO framework analysis help in evaluating a companys competencies?
-
Why do some employees of an organization behave unethically? Why is it necessary for an organization to develop employees ethics?
-
Why is strategic control important in monitoring the process of strategy implementation?
-
The consistency concept requires the entity to give the same treatment to comparable transactions from period to period. Under what circumstances can an entity change its accounting methods, provided...
-
Wholesalers Ltd. deals in the sale of foodstuffs to retailers. Owing to economic depression, the firm intends to relax its credit policy to boost productivity and sales. The firms current credit...
-
An alkyne with molecular formula C 4 H 6 was treated with ozone followed by water to produce a carboxylic acid and carbon dioxide. Draw the expected product when the alkyne is treated with aqueous...
-
Starting with acetylene, show the reagents you would use to prepare the following compounds: (a) 1-Butyne (b) 2-Butyne (c) 3-Hexyne (d) 2-Hexyne (e) 1-Hexyne (f) 2-Heptyne (g) 3-Heptyne (h) 2-Octyne...
-
Preparation of 2,2-dimethyl-3-octyne cannot be achieved via alkylation of acetylene. Explain.
-
You have just been hired as the controller of a corporation. You have been asked to review the financials. As part of this process, you notice that there is goodwill of $5,000,000 on the books based...
-
Magenta light shines on a green shirt. What color of light would you expect to be reflected?
-
How do some executives think the customer experience could be better than that reqWhat was the goal of the Hospital Price Transparency Rule when it was put into effect in 2021 by CMS?
Smart Contracts And Comparative Law A Western Perspective 1st Edition - ISBN: 3030832392 - Free Book

Study smarter with the SolutionInn App


