Use Table 9.4 to calculate an approximate enthalpy of reaction for Table 9.4 Strategy Use Equation 9.2,
Question:
Use Table 9.4 to calculate an approximate enthalpy of reaction for![]()
Table 9.4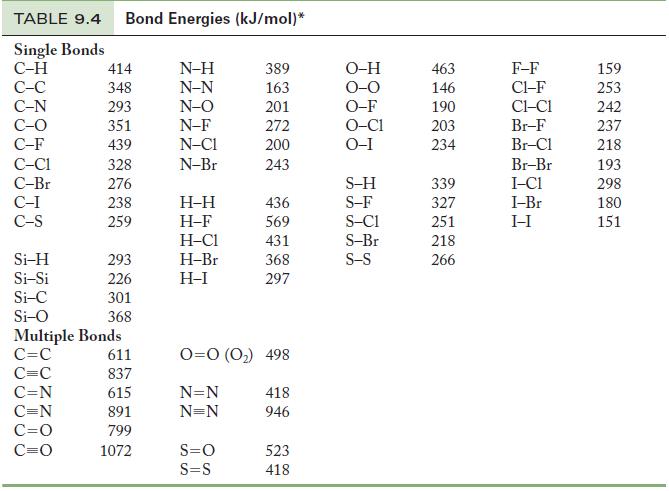
Strategy
Use Equation 9.2, being careful to substitute the correct number of moles of bonds being formed or broken. Also, write the Lewis structure of each species to determine the types (single, double, or triple) of bonds being formed or broken.
Equation 9.2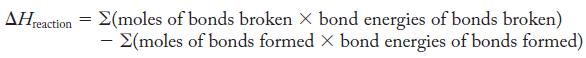
Transcribed Image Text:
CH4(g) + 2O2(g) → CO₂(g) + 2H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Three important points are 1 The Lewis structure of each compound in the reaction must be known beca...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
List major external and internal customer groups of apple Explain the value exchange for each customer group listed of apple
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Use Table 9.4 to calculate an approximate enthalpy change for (a) The combustion of 1 mol C 2 H 4 in excess molecular oxygen to form gaseous water and CO 2 . (b) The reaction of 1 mol formaldehyde, H...
-
A business wishes to have $80,000 ten years from now to make a down payment on a capital asset. How much money would the business need to invest in equal annual payments at a compound interest rate...
-
The law of radioactive decay is N(t) = N 0 e -t , where N 0 is the number of radioactive nuclei at t = 0, N(t) is the number remaining at time t, and is a quantity known as the decay constant. What...
-
Aqua-Marine manufactures fiberglass fishing boats. The manufacturing costs incurred during its first year of operations are shown as follows: Direct materials purchased . . . . . . . . . . . . . . ....
-
A mixture of \(\mathrm{H}_{2}\) and \(\mathrm{D}_{2}\) is contained in two bulbs connected by a porous plug. The bulbs are maintained at different temperatures of 200 and \(600 \mathrm{~K}\). The...
-
Justification/Recommendation Report: Improving Greenhouse Markets Service You are a recently hired manager for Greenhouse Market, a highend fast-food restaurant that has been in business for three...
-
Given points A(6,-2,1) B(0,5,6) and C(-3,-5,2) a) Find the vector equation of the line through A and B b) Find the distance from point C to line in part a) c) Determine the scalar equation of the...
-
Describe what is meant by resonance structures.
-
Which elements can form expanded valence shell molecules? Do the two xenon compounds shown below have an expanded valence shell? Explain your answer. XeF2 XeF4
-
Give some examples of each type of cost for a hospitals emergency services department.
-
Maher Company's records for May contained the following information: Direct materials purchased Direct materials used $ 16,000 $14,000 Actual direct labor cost $ 47,000 Actual direct labor hours...
-
A company uses the weighted-average method in its process costing system. Operating data for the first processing department for the month of June appear below: WIP - June 1 Started uring June WIP...
-
On 1 January 2 0 2 2 solutions Ltd purchased 5 0 0 0 debentures in kappa Ltd at a discount of 1 0 % on the face value of R 5 0 per debenture. The market - related interest rate on similar debentures...
-
I: (Horizontal analysis - Increase /Decrease Method) Following is the comparative statement of financial position of SPINACH Company: NIVS Current Assets Plant Assets Total Assets C SPINACH COMPANY...
-
A company has liabilities of $ 1 5 , 5 0 0 and $ 2 0 , 2 0 0 of owners' equity at the year end. What is the total amount of assets held by the firm at the year end? $ 4 , 7 0 0 $ 3 5 , 7 0 0 $ 2 0 ,...
-
If a competitive firm is paying $8 per hour (with no fringe benefits) to its employees, what would tend to happen to its equilibrium wage if the company began to give on-the-job training or free...
-
Anne is employed by Bradley Contracting Company. Bradley has a $1.3 million contract to build a small group of outbuildings in a national park. Anne alleges that Bradley Contracting has discriminated...
-
Draw the hypothetical ring-flip of trans-decalin, and explain why it does not occur. Use this analysis to explain why cholesterol has a fairly rigid three-dimensional geometry.
-
Prednisolone acetate is an anti-inflammatory agent in clinical use. It is similar in structure to cortisol, with the following two differences: (1) Prednisolone acetate exhibits a double bond between...
-
Classify each prostaglandin according to the instructions provided in Section 26.7. In section 26.7 ¢ Prostaglandins are biochemical regulators that are even more powerful than steroids. ¢...
-
Q) Define what an access control list (ACL) is and describe a way it is used to provide security in a network. Include a specific example of ACL use.
-
Recently, a disgruntled employee was fired from the company, but before they left, they were able to lock network administrators out of several vital networking devices. What process failed? How can...
-
11. (6%) BigCo has been allocated IP address block 66.6.0.0/24 for use on their corporate network. They have these subnet requirements: Reception: 4 IP addresses IT Staff: 70 IP addresses Executive:...

Study smarter with the SolutionInn App


