Using the following thermochemical equations 7 CH6(g) + O(g)2CO(g) + 3HO(l) 2CH(g) + 50(g) 4CO2(g) +2HO(l)
Question:
Using the following thermochemical equations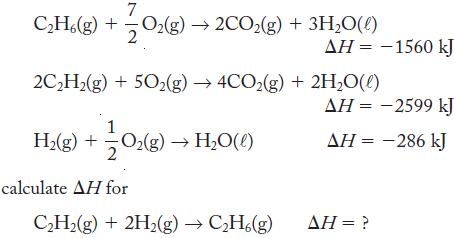
Transcribed Image Text:
7 C₂H6(g) + O₂(g)→2CO₂(g) + 3H₂O(l) 2C₂H₂(g) + 50₂(g) → 4CO2(g) +2H₂O(l) H₂(g) + 0₂(g) → H₂O(l) calculate AH for AH = -1560 kJ C₂H₂(g) + 2H₂(g) → C₂H6(g) ΔΗ = -2599 kJ ΔΗ = -286 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation of ethylene to form ethane. Calculate the enthalpy change for Use the following...
-
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to...
-
Refer to the RMO CSMS Order Fulfillment subsystem shown in Figure. Draw a use case diagram that shows all actors and all use cases. Use a drawing tool such as Microsoft Visio if it is available.
-
The sucrose in ground coffee particles of an average diameter of 2 mm is to be extracted with water in a continuous, Counter current extractor at 25C. The diffusivity of the sucrose in the particles...
-
a. Prove that e x 1 + x if x 0. b. Use the result in part (a) to show that et 1 + x + 2.1.
-
On 1 June 2026, Aaron Draper began trading as a landscape material supplier. The transactions for the month of June 2026 were as follows (ignore GST): The following additional information is...
-
Coakley Company acquired 30% of the outstanding common stock of Ginger Inc. on January 1, 2012, by paying $1,800,000 for 60,000 shares. Ginger declared and paid a $0.50 per share cash dividend on...
-
23. A convex mirror used for rear view on an automobile has a radius of curvature of 3m. If a bus is located at 5m from this mirror, find the position, nature and magnification of the image. 24. An...
-
You are part of the engagement team for the audit of Suzuki Manufacturing for the year ended December 31, 2019, and are responsible for auditing the acquisition cycle. Download the Excel file for the...
-
Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following thermochemical equations CH4(g) + 20(g) CO(g) + 2HO(l) CH4(g) + 30(g) 2CO(g) + 2HO(l)...
-
Draw an energy-level diagram based on each of the following thermochemical equations. Label each level with the amounts of substances present, and use an arrow between levels for the given enthalpy...
-
Explain the actions of the implode and explode functions.
-
Calculate the lower heat of reaction at constant volume for benzene \(\mathrm{C}_{6} \mathrm{H}_{6}\) at \(25^{\circ} \mathrm{C}\). The heats of formation at \(25^{\circ} \mathrm{C}\) are: benzene,...
-
The products of combustion of a jet engine have a molecular weight, \(m_{w}\), of 30 and a molar specific heat at constant pressure given by \(c_{p, m}=3.3 \times 10^{4}+15 T \mathrm{~J} /...
-
Working in pairs, have one person describe a time when someone didnt listen well. This can be a situation with a family member, friend, doctor, or someone else. Have the listener identify (1) What...
-
A jet engine burns a weak mixture of octane \(\left(\mathrm{C}_{8} \mathrm{H}_{18} ight)\) and air, with an equivalence ratio, \(\phi=2\). The products of combustion, in which dissociation may be...
-
Distinguish between an ideal and a perfect gas and show that in both cases the specific entropy, \(s\), is given by \[s=s_{0}+\int_{T_{0}}^{T} \frac{\mathrm{d} h}{T}-\Re \ln \left(\frac{p}{p_{0}}...
-
Suppose that you buy a two-year 8% bond at its face value. a. What will be your nominal return over the two years if inflation is 3% in the first year and 5% in the second? What will be your real...
-
Trade credit from suppliers is a very costly source of funds when discounts are lost. Explain why many firms rely on this source of funds to finance their temporary working capital.
-
From the data in Table 10.3 (see Appendix B, Data Tables), calculate the activity of the electrolyte in 0.200 m solutions assuming complete dissociation of a. KCl b. Na 2 SO 4 c. MgCl 2
-
Estimate the degree of dissociation of a 0.200 m solution of nitrous acid (K a = 4.00 10 4 ) that is also 0.500 m in the strong electrolyte given in parts (a)(c). Use the data tables to obtain ,...
-
Calculate H o R and G o R for the reaction Ba(NO 3 ) 2 (aq) + 2KCl(aq) BaCl 2 (s) + 2KNO 3 (aq).
-
How does the cell cycle, a highly regulated process governing the sequential progression of cells through interphase, mitosis, and cytokinesis, ensure faithful duplication of genetic material,...
-
An injury insurance policy pays out $6000 if a person is seriously injured with permanent disability, $1000 if a person is seriously injured without permanent disability and $0 otherwise. The clients...
-
Which is true of the null model in a regression analysis? It is only accepted when the p value is less than 0.05 It assumes that the slope of the line is zero (0) It is calculated by the change in...

Study smarter with the SolutionInn App


