What does an energy-level diagram for the reverse reaction from Exercise 5.71 look like? Exercise 5.71 Given
Question:
What does an energy-level diagram for the reverse reaction from Exercise 5.71 look like?
Exercise 5.71
Given the thermochemical equations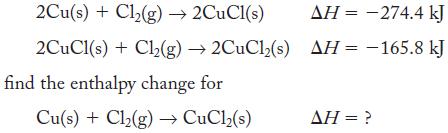
Transcribed Image Text:
2Cu(s) + Cl₂(g) → 2CuCl(s) 2CuCl(s) + Cl₂(g) →2CuCl2(s) find the enthalpy change for Cu(s) + Cl₂(g) → CuCl₂(s) AH = -274.4 kJ AH = -165.8 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Cus Clg 137...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What does an energy-level diagram for the reverse reaction from Exercise 5.72 look like? Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
Draw an energy level diagram for the chemical reaction in Exercise 9. In exercise 9, Nitrogen gas reacts with oxygen gas to make NO(g) while absorbing 180 kJ. Is this process exothermic or...
-
This question is about endothermic and exothermic reactions. a. Explain the meaning of the terms endothermic and exothermic. b. (i) Draw an energy level diagram for the reaction: (ii) Is this...
-
Suppose that a telephone switchboard of some company on the average handles 300 calls per hour, and that the board can make at most 10 connections per minute. Using the Poisson distribution, estimate...
-
A laboratory screen analysis for a batch of crystals of hypo (sodium thiosulfate) is as follows. Prepare both differential and cumulative-undersize plots of the data, using a spreadsheet. In...
-
Right, or wrong? Give a brief reason why. -15(x + 3) (x - 2)4 -dx x + (x - 2) 3 + C
-
On 1 July 2025 Roxanne and Asterios formed a partnership with initial capital balances of \($280\)000 and \($310\)000 respectively. The Profit or Loss Summary account for the year ended 30 June 2026...
-
Emarpy Appliance is a company that produces all kinds of major appliances. Bud Banis, the president of Emarpy, is concerned about the production policy for the companys best-selling refrigerator. The...
-
Which one of the metal atoms whose electronic structure given below forms a nitrate of the type M(NO3)2? 37 A. 2.8.0 B. 2.8.1 C. 2.8.2 D. 2.8.3
-
If the resultant force acting on the bracket is FR = {? 300i + 650j + 250k} N, determine the magnitude and coordinate direction angles of F. 45 30- F= 750 N
-
Write the formation reaction for each of the following substances. (a) HBr(g) (b) HSO4(l) (c) 03(g) (d) NaHSO4(s)
-
Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.72. Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
As a jewelry store manager, you want to offer credit, with interest on outstanding balances paid monthly. To carry receivables, you must borrow funds from your bank at a nominal 9%, monthly...
-
Compute the mean FEV1 for those with asthma and those without. What is counterintuitive about the result?
-
depicts a closed cycle gas turbine operating on the Joule cycle (i.e. constant pressure heat addition and rejection, and isentropic compression and expansion). Energy is added to the working fluid...
-
An amount of pure substance equal to \(1 \mathrm{kmol}\) undergoes an irreversible cycle. Neglecting the effects of electricity, magnetism and gravity, state whether each of the following...
-
A thermally isolated system at constant pressure consists of \(10 \mathrm{~kg}\) of air at a temperature of \(1000 \mathrm{~K}\) and \(10 \mathrm{~kg}\) of water at \(300 \mathrm{~K}\), connected...
-
A system at constant pressure consists of \(10 \mathrm{~kg}\) of air at a temperature of \(1000 \mathrm{~K}\). Calculate the maximum amount of work which can be obtained from the system if the...
-
Company Zs earnings and dividends per share are expected to grow indefinitely by 5% a year. If next years dividend is $10 and the market capitalization rate is 8%, what is the current stock price?
-
The landing gear of an aircraft with: mass of 2000 kg the spring-mass-damper system Consider that the runway surface is y(t) = 0.2 cos 157.08t stiffness of the spring is 5 x 105 N/m. What is the...
-
Why is it not possible to measure the activity coefficient of Na + (aq)?
-
Why is it possible to formulate a general theory for the activity coefficient for electrolyte solutions, but not for Non-electrolyte solutions?
-
Why does an increase in the ionic strength in the range where the DebyeHckel law is valid lead to an increase in the solubility of a weakly soluble salt?
-
Imagine that you are an Associate at a private equity firm that focuses on investments in the Financial Services space. In the Excel file, you will find an Income Statement for an insurance broker....
-
An object is pushed from rest across a sheet of ice, accelerating at 5.0 m/s over a distance of 80.0 cm. The object then slides with a constant speed for 4.0 s until it reaches a rough section which...
-
Linda is saving for retirement and would like to accumulate $800,000 at her retirement. She currently has $30,000 saved and would like to work for another 25 years. She plans to save $3500 at the end...

Study smarter with the SolutionInn App


