What does an energy-level diagram for the reverse reaction from Exercise 5.72 look like? Exercise 5.72 In
Question:
What does an energy-level diagram for the reverse reaction from Exercise 5.72 look like?
Exercise 5.72
In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: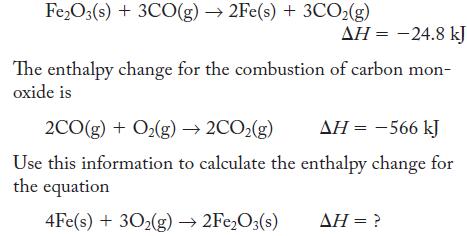
Transcribed Image Text:
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) ΔΗ = -24.8 kJ The enthalpy change for the combustion of carbon mon- oxide is 2CO(g) + O₂(g) → 2CO₂(g) AH = -566 kJ Use this information to calculate the enthalpy change for the equation 4Fe(s) + 302(g) → 2Fe₂O3(s) AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
has to be determined for the reaction Concept Introduction According to Hesss Laws change in an over...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.72. Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO(g) = -24.8 kJ The enthalpy change...
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
Create the GUI in Fig. 14.2 (you do not have to provide functionality). Figure 14.2 Printer GUI Ok Printer. Bugalicious X9000 O Image O Selection Cancel Text O Current page Code ComboBox Setup....
-
Derive expressions for the surface-mean and mass-mean diameter from a particle-size analysis based on counting, rather than weighing, particles in given size ranges, letting N i be the number of...
-
The 8-ft wall shown here stands 27 ft from the building. Find the length of the shortest straight beam that will reach to the side of the building from the ground outside the wall. Beam 8' wall -27'-...
-
Victor and Vincents partnership had a final profit for the year of \($40\) 500. When the partnership was formed at the beginning of the year Victor invested \($150\) 000 and Vincent invested \($100\)...
-
On June 30, Petrov Co. has $ 128,700 of accounts receivable. Prepare journal entries to record the following selected July transactions. Also prepare any footnotes to the July 31 financial statements...
-
22 22 The atomic number of an element T is 10. T shows similar properties to an element with atomic number 23. 24. A. 14 B. 16 C. 18 d. 20 The electron configurations of elements W, X, Y and Z are...
-
On December 1, Year 1, John and Patty Driver formed a corporation called Susquehanna Equipment Rentals. The new corporation was able to begin operations immediately by purchasing the assets and...
-
Write the formation reaction for each of the following substances. (a) HBr(g) (b) HSO4(l) (c) 03(g) (d) NaHSO4(s)
-
What does an energy-level diagram for the reverse reaction from Exercise 5.71 look like? Exercise 5.71 Given the thermochemical equations 2Cu(s) + Cl(g) 2CuCl(s) 2CuCl(s) + Cl(g) 2CuCl2(s) find the...
-
The Chronicle of Higher Education (January 13, 1993) reported that 72.1% of those responding to a national survey of college freshmen were attending the college of their first choice. Suppose that n...
-
Recalculate P3.11 with the pressure at which steam is reheated and reduced to 5 bar. What have been the benefits of using this lower pressure? [32.36\%; 51.40\%; \(1101 \mathrm{~kW} /(\mathrm{kg} /...
-
Air enters the compressor of a simple gas turbine at a pressure of \(1 \mathrm{bar}\) and a temperature of \(25{ }^{\circ} \mathrm{C}\). The compressor has a pressure ratio of 15 , and an isentropic...
-
Using the FRED website, chart the level of M1 and M2. Chart 12month growth rates for each. What might you infer about the performance of the economy based on these growth rates?
-
A steam turbine operates on a superheated Rankine cycle. The pressure and temperature of the steam leaving the boiler are 10 bar and \(350^{\circ} \mathrm{C}\) respectively. The specific steam...
-
The condenser pressure of the turbine in P3.2 is reduced to 0.15 bar. Calculate the same parameters for this cycle as in the previous example. Why have the parameters improved so much? [28.97\%;...
-
Company Z-prime is like Z in all respects save one: Its growth will stop after year 4. In year 5 and afterward, it will pay out all earnings as dividends. What is Z-primes stock price? Assume next...
-
XYZ Inc. a calendar year, accrual basis corporation, had the following items during 2021: Gross revenue from operations Cost of goods sold $420,000 ($180,000) $9,000 LT capital gain .LT capital...
-
How is the mean ionic chemical potential of a solute related to the chemical potentials of the anion and cation produced when the solute is dissolved in water?
-
How is the chemical potential of a solute related to its activity?
-
Tabulated values of standard entropies of some aqueous ionic species are negative. Why is this statement not inconsistent with the third law of thermodynamics?
-
The Fresh Turkey Company produces two types of turkey cutlets for sale to fast-food restaurants. Each type of cutlet consists of white meat and dark meat. Cutlet 1 sells for $2.97 a pound and must...
-
6) One business has a large amount of debt on which it pays an annual yield of 8%. A competitor has no debt, and any excess money goes into the bank and earns a yield of 2.3%. Each of these...
-
Jaycee estimates that the repairs to a Cadillac Escalade damaged in an accident will take 45 hours of labor and $3,500 in parts and materials. The total cost of the repairs is ? The price used to...

Study smarter with the SolutionInn App


