What is the fraction of acid ionized in each acid in Exercise 15.64? Exercise 15.64 Use the
Question:
What is the fraction of acid ionized in each acid in Exercise 15.64?
Exercise 15.64
Use the Ka values in Table 15.6 to calculate the pH of the following solutions.
(a) 0.050 M HI
(b) 0.85 M HF
(c) 0.15 M CH3COOH
(d) 0.017 M C6H5COOH
Table 15.6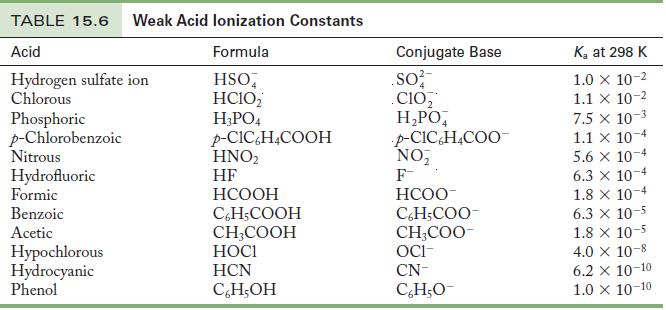
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The fraction of acid ionized often represented as a can be calculated using the ionization constant ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
278+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is the fraction of acid ionized in each acid in Exercise 15.63? Exercise 15.63 Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.33 M HNO 2 (b)0.016 M...
-
What is the fraction of acid ionized in each acid in Exercise 15.62? Exercise 15.62 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
What is the fraction of acid ionized in each acid in Exercise 15.61? Exercise 15.61 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
} S 1995 the # of Farms Century, data year 1935 1990 19.50 Aumcon people living on declined steadily during the shown by the Follow g as (in milion of persons) from 1935 19.55 1960 1965 11975 1980...
-
A concentric tube heat exchanger uses water, which is available at 15C, to coo] ethylene glycol from 100 to 60C. The water and glycol flow rates are each 0.5 kg/s. What are the maximum possible heat...
-
Consider the ideal Otto, Sterling, and Carnot cycles operating between the same temperature limits. How would you compare the thermal efficiencies of these three cycles?
-
Event A: Randomly select a U.S. citizen of Indian origin.. Event B: Randomly select a U.S. citizen of Chinese origin. Determine whether the events are mutually exclusive. Explain your reasoning.
-
Business Objects trades on the Paris Bourse as ordinary shares and on the NASDAQ as American Depositary Receipts (ADRs). One ADR of Business Objects corresponds to one share on the Paris Bourse....
-
You would like to calculate the benefits of one of the training programs in your organization. The cost of the program is $38,000 and the benefit is $205,000. Using this information, calculate each...
-
Write the chemical equation for the ionization of caffeine, a weak base. The chemical formula of caffeine is C 8 H 10 N 4 O 2 .
-
Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.050 M HI (b)0.85 M HF (c)0.15 M CH 3 COOH (d) 0.017 M C 6 H 5 COOH Table 15.6
-
What is a null value? What gives rise to null values in a relation?
-
Until a year ago, your business regularly used a local stationery and printing service. They were not that reliable, but you wanted to support a local business. One day, they suddenly informed you...
-
Are promotional slogans such as Best sushi in town or Atlantas most popular restaurant ethical? Why or why not?
-
An ethical __________ exists when a person is faced with conflicting but ethical choices or alternatives that are neither entirely right nor entirely wrong; an ethical __________ occurs when a person...
-
Shari Willison worked as a geologist in your civil engineering firm for 20 years before succumbing to leukemia last week. With only a few dozen employees, the company has always been a tight-knit...
-
Working as an associate lecturer for a well-known and successful online university, you use your own home as your office. Periodically, you teach residential courses and short, locally based...
-
What are the reasons that the cost of poor quality reached an epidemic level before U.S. companies were motivated to do something about the problem in the 1980s?
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
This problem involves the design of a parallel adder-subtracter for 8-bit numbers expressed in sign and magnitude notation. The inputs X and Y are in sign and magnitude, and the output Z must be in...
-
Design a multiplier that will multiply two 16-bit signed binary integers to give a 32-bit product. Negative numbers should be represented in 2s complement form. Use the following method: First...
-
The objective of this problem is to use Verilog to describe and simulate a multiplier for signed binary numbers using Booths algorithm. Negative numbers should be represented by their 2s complement....
-
Let a = ( - 2 , 2 , 1 ) , b = ( - 2 , 0 , 1 ) , c = ( 0 , 1 , - 3 ) , d = ( , 1 - 3 , - ) , and e = ( 1 , - 2 , - ) be any five vectors in R 3 . Find if d is a unit vector.
-
6) Divide the fundamental Frequency of F by the frequency of A4 in the following tuning systems. Round your answers (if necessary) to the nearest 0.000001: a) Equal Temperament Tuning b) Harmonic...
-
Question 3 [6 marks] When the brakes on a car are put on, a car decelerates at the uniform rate of -6 m/sec. If it initially had a speed of 45 m/sec, then what will its speed be after 4 seconds have...

Study smarter with the SolutionInn App


