Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a)
Question:
Use the Ka values in Table 15.6 to calculate the pH of the following solutions.
(a) 0.050 M HI
(b) 0.85 M HF
(c) 0.15 M CH3COOH
(d) 0.017 M C6H5COOH
Table 15.6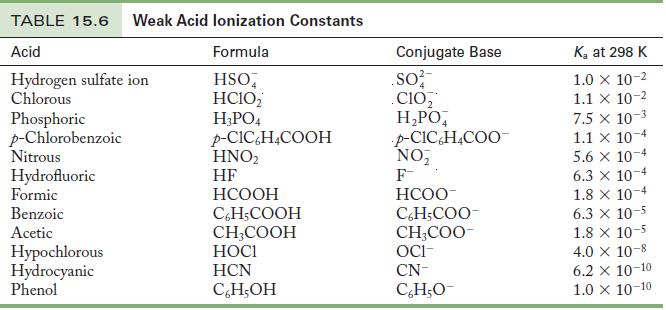
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
General Steps Identify the relevant acid for each solution from the question Locate the correspo...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1 . Which of the following is a device --------------- a .water filter b. mouse c. air conditioning 2. is a unit of physical ---------------- hardware or equipment that provides one or more computing...
-
Don works as a financial adviser in a practice with three other advisers. They each own 25% of the business and while they each look after their own clients, they do share back office, paraplanning...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Evaluate the integral (4e* + 2 In (2))dx.
-
A shell-and-tube heat exchanger with one shell pass and 20 tube passes uses hot water on the tube side to heat oil on the shell side. The single copper tube has inner and outer diameters of 20 and 24...
-
For what compressor efficiency will the gas-turbine power plant in Problem 980E produce zero net work?
-
You are given that P(A) = 0.35 and P(B) = 0.25. Do you have enough information to find P(B) and P(A and B)? Explain. Determine whether the events are independent or dependent. Explain your reasoning.
-
A corporation has $5,000,000 of 10 percent bonds and $3,000,000 of 12 percent of preferred stock. The firms financial break even (assuming 40 percent tax rate) is? 1. 860K 2. 716K 3. 1.4MIL 4. 1.1MIL
-
Calculate and provide an analysis of the VAR for C. H. Robinson Worldwide in 2021. What does this tell you about your company in terms of risk? Reference:...
-
What is the fraction of acid ionized in each acid in Exercise 15.61? Exercise 15.61 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.33 M HNO 2 (b)0.016 M phenol, C 6 H 5 OH (c)0.25 M HF (d) 0.010 M HCOOH Table 15.6
-
In a survey of 2096 U.S. adults, 1740 think football teams of all levels should require players who suffer a head injury to take a set amount of time off from playing to recover. (a) Find the point...
-
As a motivated, ambitious employee, you naturally care about your performance on the joband about making sure your performance is being fairly judged and rewarded. Unfortunately, the company has gone...
-
You work for a large, publicly owned organization that procures many services from organizations based both nationally and more locally to your offices. For many years your offices have been supplied...
-
Analyze the strengths and weaknesses of this message, and then revise it so that it follows this chapters guidelines for sharing routine information, including using the direct approach: Those of you...
-
A carefully constructed series of tweets can serve as a summary of a blog post, video, or other message or document. Your task: Find any article, podcast, video, or webpage on a business topic that...
-
Your company markets a line of rugged smartphone cases designed to protect the sensitive devices from drops, spills, and other common accidents. Your guarantee states that you will reimburse...
-
Paragon Manufacturing produces small motors for assembly in handheld tools such as chain saws and circular saws. The company recently began manufacturing a new motor, model EZ3, and forecasts an...
-
Identify the source of funds within Micro Credit? How does this differ from traditional sources of financing? What internal and external governance mechanisms are in place in Micro Credit?
-
A block diagram for a 16-bit 2s complement serial subtracter is given here. When St = 1, the registers are loaded and then subtraction occurs. The shift counter, C, produces a signal C15 = 1 after 15...
-
(a) Figure 4-12 shows the block diagram for a 32-bit serial adder with accumulator. The control circuit uses a 5-bit counter, which outputs a signal K = 1 when it is in state 11111. When a start...
-
Write Verilog code for a shift register module that includes a 16-bit shift register, a controller, and a 4-bit down counter. The shifter can shift a variable number of bits depending on a count...
-
At $6.75 per square foot, plus $60 delivery charge, how much will it cost to purchase artificial turf for a square lawn 24 feet 6 inches on a side? Round your answer to the nearest cent ( hundredth )
-
A tour operator believes that the profit P , in dollars, from selling x tickets is given byP ( x ) = 4 5 x - 0 . 2 5 x ^ 2 . Using this model, what is the maximum profit the tour operator can expect?
-
An 2Bm (b being a positive number) meter ladder is leaned against a church wall. A cat is tied to the bottom of the ladder. It spots a mouse and moves towards it at a constant speed of 2m/s, pulling...

Study smarter with the SolutionInn App


