When 0.030 mol HCl dissolves in 100.0 g benzene, the solution freezes at 4.04 C. When 0.030
Question:
When 0.030 mol HCl dissolves in 100.0 g benzene, the solution freezes at 4.04 °C. When 0.030 mol HCl dissolves in 100.0 g water, the solution freezes at -1.07 °C. Use the data in Table 12.4 to complete the following exercises.
Table 12.4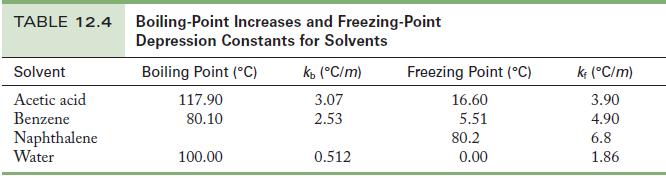
(a) From the freezing point, calculate the molality of HCl in the benzene solution.
(b) Use the freezing point of the aqueous solution to find the molality of HCl in the water.
(c) Offer an explanation for the different values found in parts a and b.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





