Consider the formation of ammonia from the elements: (a) If the ammonia concentration is increasing at a
Question:
Consider the formation of ammonia from the elements:![]()
(a) If the ammonia concentration is increasing at a rate of 0.024 M/s, what is the rate of reaction?
(b) What is the rate of disappearance of hydrogen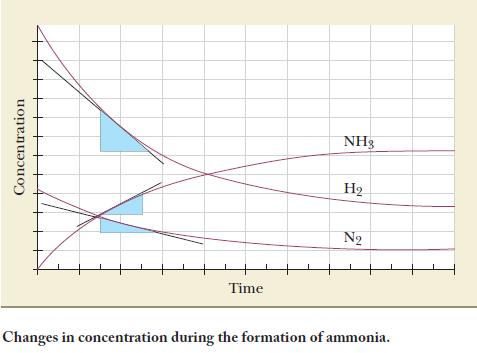
Strategy
Use the rate of change of ammonia and the stoichiometry of the chemical equation to compute the rate of reaction. Write the chemical equation and then divide by the appropriate coefficient in the equation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





