When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay. The
Question:
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay.![]()
The rate of reaction is monitored by measuring the total pressure in the reaction container.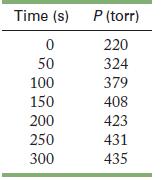
Calculate the rate constant and half-life, in seconds, for the reaction. At the start of the reaction (time = 0), only formic acid is present.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





