Question: Write a balanced equation for the reaction pictured below. In the diagrams, the red spheres represent oxygen atoms and blue spheres represent nitrogen atoms. Strategy
Write a balanced equation for the reaction pictured below. In the diagrams, the red spheres represent oxygen atoms and blue spheres represent nitrogen atoms.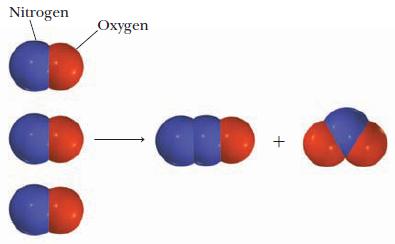
Strategy
Examine the diagram to determine the formula of each molecule; then count the number of molecules of each type in the picture and use those numbers as the coefficients of the equation.
Nitrogen Oxygen +
Step by Step Solution
★★★★★
3.47 Rating (144 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The left reactant side con... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


