Question: A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential Zn(s) = -0.763 V Zn+ (aq)+2e crom (aq)+4 H20(1)+3e ,
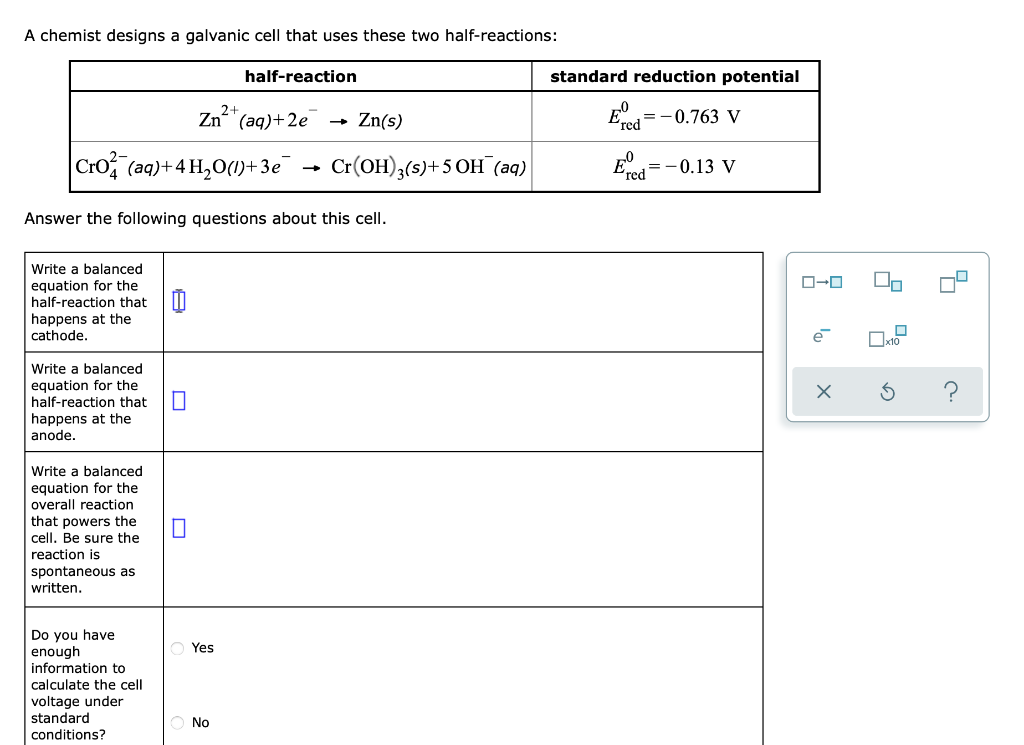
A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential Zn(s) = -0.763 V Zn+ (aq)+2e crom (aq)+4 H20(1)+3e , Cr(OH)3(s)+50H (aq) Ered = -0.13 V Answer the following questions about this cell. 1. so Write a balanced equation for the half-reaction that 0 happens at the cathode. lo x10 X Write a balanced equation for the half-reaction that happens at the anode. 5 ? Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written D Yes Do you have enough information to calculate the cell voltage under standard conditions? No Write a balanced equation for the half-reaction that happens at the cathode. e xo $ Write a balanced equation for the half-reaction that happens at the anode. D ? Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Yes Do you have enough information to calculate the cell voltage under standard conditions? No If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 2 significant digits. v
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


