Consider the ionic compounds KF, NaCl, NaBr, and LiCl. (a) Use ionic radii (Figure 7.8) to estimate
Question:
Consider the ionic compounds KF, NaCl, NaBr, and LiCl.
(a) Use ionic radii (Figure 7.8) to estimate the cation–anion distance for each compound.
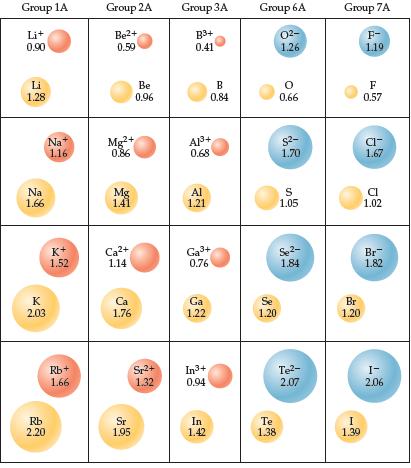
(b) Based on your answer to part (a), arrange these four compounds in order of decreasing lattice energy.
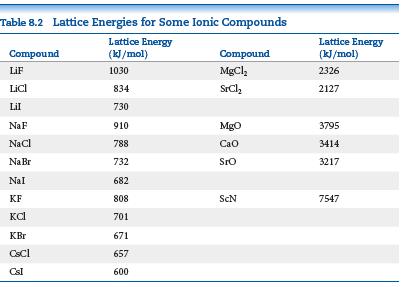
(c) Check your predictions in part (b) with the experimental values of lattice energy from Table 8.2. Are the predictions from ionic radii correct?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





