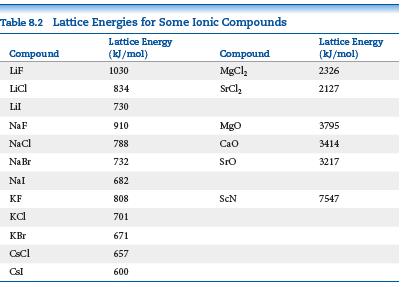
The ionic substances NaF, CaO, and ScN are isoelectronic (they have the same number of electrons). Examine
Question:
The ionic substances NaF, CaO, and ScN are isoelectronic (they have the same number of electrons). Examine the lattice energies for these substances in Table 8.2. Make a graph of lattice energy on the vertical axis versus the charge on the cation on the horizontal axis.
(a) What is the slope of the line?
(b) Make a graph of lattice energy on the vertical axis versus the square of the cation charge on the horizontal axis. What is the slope of this line?
(c) Compare how well the data points fall on a line for the graphs in (a) and (b). Which trend is more linear, lattice energy versus cation charge or lattice energy versus cation charge squared?
(d) Predict the lattice energy for the compound TiC, if we consider the carbon to have a 4- charge.
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





