Consider the Lewis structure for glycine, the simplest amino acid: (a) What are the approximate bond angles
Question:
Consider the Lewis structure for glycine, the simplest amino acid:
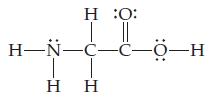
(a) What are the approximate bond angles about each of the two carbon atoms, and what are the hybridizations of the orbitals on each of them?
(b) What are the hybridizations of the orbitals on the two oxygens and the nitrogen atom, and what are the approximate bond angles at the nitrogen?
(c) What is the total number of s bonds in the entire molecule, and what is the total number of p bonds?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





