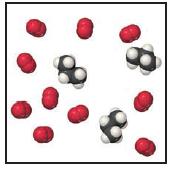
Consider the mixture of propane, C 3 H 8 , and O 2 shown below. (a) Write
Question:
Consider the mixture of propane, C3H8, and O2 shown below.
(a) Write a balanced equation for the combustion reaction that occurs between propane and oxygen.
(b) Which reactant is the limiting reactant?
(c) How many molecules of CO2, H2O, C3H8, and O2 will be present if the reaction goes to completion?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
a The given combustion reaction between propane and oxygen CH 3 H 8 g O 2 g CO 2 g H 2 Og Let us bal...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
Sunburts distributes hardcover books to retail stores. At the beginning of June, 2021, the ledger of Sunburts showed Cash of $3,000 and Share Capital Ordinary of $3,000. Jun 1 Purchased books on...
-
A gas mixture with a molar analysis of 20% C3H8 (propane) and 80% air enters a control volume operating at steady state at location 1 with a mass flow rate of 5 kg/min, as shown in Fig. P12.10. Air...
-
Propane C3H8 is burned with air, and the combustion products consist of CO2, CO, H2O, O2, N2, OH, H2, and NO. The number of equilibrium constant relations needed to determine the equilibrium...
-
A taxpayer has qualified business income of $50,000 from partnership A and a qualified business loss of $30,000 from partnership B. Wages allocable to the taxpayers interest in partnership A were...
-
The Honda Civic Hybrid vehicle, powered by gasoline-electric technology, gets 49 miles per gallon (mpg) in city driving and 51 mpg in highway driving (Source: Honda.com). The car is driven 447 mi on...
-
With the members of your team, obtain the first lines of the amortization table for a mortgage loan of $800,000.00. Consider monthly payments for 5 years, an interest rate of 20.4% nominal monthly...
-
Consider the multiple linear regression model fit to the rental price data in Problem 3.42. Problem 3.42 Table B.24 contains data on median family home rental price and other data for 51 US cities....
-
Peter Johnson, the CFO of Homer Industries, Inc. is trying to determine the Weighted Cost of Capital (WACC) based on two different capital structures under consideration to fund a new project. Assume...
-
ExplainCapital Structure and give an example.Define Capital Structure and give an example.
-
Brads Bicycle Shop sells 21-speed bicycles. For purposes of a cost- volume-profit analysis, t he shop owner has divided sales into two categories, as follows: Seventy percent of the shops sales are...
-
Consider the mixture of ethanol, C 2 H 5 OH, and O 2 shown in the accompanying diagram. (a) Write a balanced equation for the combustion reaction that occurs between ethanol and oxygen. (b) Which...
-
The nucleus of 6Li is a powerful absorber of neutrons. It exists in the naturally occurring metal to the extent of 7.5%. In the era of nuclear deterrence, large quantities of lithium were processed...
-
In the preparation of ethynylmagnesium bromide by the transmetallation reaction of Eq. 14.24, ethylmagnesium bromide is added to a large excess of acetylene in THF solution. Two side reactions that...
-
During the 1940s and 1950s, due to the wage freeze, companies expanded their discretionary benefits as an alternative to wage increases. true. false
-
Jon just purchased a security which he describes this way: " My security allows me to buy stock in Company XYZ for $ 2 5 per share any time between now and 9 0 days from now". What does the $ 2 5...
-
According to the video, what is the focus of Chapter 1 1 ? a Comparing nonprofit versus for - profit firms with respect to the need to engage customers globally b Comparing small firms with large...
-
Which of the following adjustment to core compensation are NOT related to the actual job performance? 1) Seniority pay: 2) Merit pay; 3) Incentive pay; 4) Person- focused pay 1) and 2) only 2) and 3)...
-
Stock Q trades at $ 3 1 a share. How would we describe the "moneyness" of a call option on Stock Q that has a strike price of $ 3 0 per share? A . Without money B . At - the money C . Out - of - the...
-
Let X denote the courtship time for a randomly selected female-male pair of mating scorpion flies (time from the beginning of interaction until mating). Suppose the mean value of X is 120 min and the...
-
What are current assets and current liabilities? How are they different from non-current assets and non-current liabilities?
-
(a) What do ethane and ethanol have in common? (b) How does 1-propanol differ from propane?
-
Chloropropane is a compound derived from propane by substituting Cl for H on one of the carbon atoms. (a) Draw the structural formulas for the two isomers of chloropropane. (b) Suggest names for...
-
The following diagram represents an ionic compound in which the red spheres represent cations and blue spheres represent anions. Which of the following formulas is consistent with the drawing: KBr,...
-
What are the main ethical issues that researchers may encounter when conducting business research and how can they address these issues ?
-
How has womens participation in the Olympics changed over the years? Explain
-
What is the Christian view of work according to the God is Not One: The Eight Rival Religions That Run the World.

Study smarter with the SolutionInn App


