Cyclopropane is an interesting hydrocarbon. Instead of having three carbons in a row, the three carbons form
Question:
Cyclopropane is an interesting hydrocarbon. Instead of having three carbons in a row, the three carbons form a ring, as shown in this perspective drawing (see Figure 2.17 for a prior example of this kind of drawing):
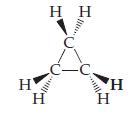 Cyclopropane was at one time used as an anesthetic, but its use was discontinued, in part because it is highly inflammable.
Cyclopropane was at one time used as an anesthetic, but its use was discontinued, in part because it is highly inflammable.
(a) What is the empirical formula of cyclopropane? How does it differ from that of propane?
(b) The three carbon atoms are necessarily in a plane. What do the different wedges mean?
(c) What change would you make to the structure shown to illustrate chlorocyclopropane? Are there isomers of chlorocyclopropane?
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





