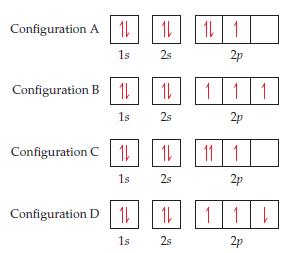
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the
Question:
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. Which one is the correct electron configuration? Which configurations violate the Pauli exclusion principle? Which configurations violate Hund’s rule?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





