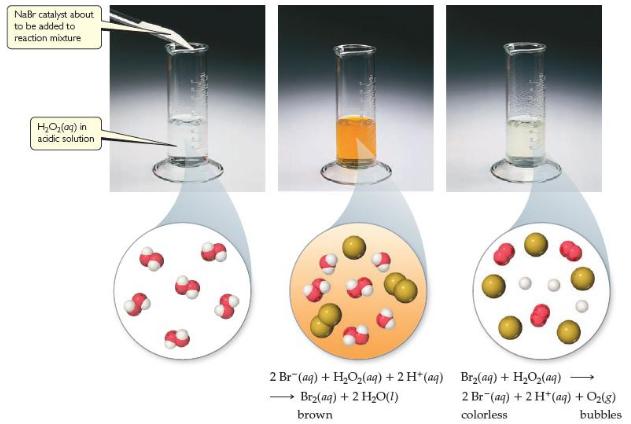
In Figure 14.22, we saw that Br - (aq) catalyzes the decomposition of H 2 O 2
Question:
In Figure 14.22, we saw that Br - (aq) catalyzes the decomposition of H2O2(aq) into H2O(l) and O2(g). Suppose that some KBr(s) is added to an aqueous solution of hydrogen peroxide. Make a sketch of [Br - (aq)] versus time from the addition of the solid to the end of the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:




