Question: Predict how each molecule or ion would act, in the Brnsted- Lowry sense, in aqueous solution by writing acid, base, both, or neither on the
Predict how each molecule or ion would act, in the Brønsted- Lowry sense, in aqueous solution by writing “acid,” “base,” “both,” or “neither” on the line provided.
(a) HCO3-, the bicarbonate ion: ______
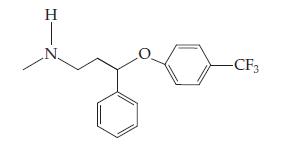
(b) Prozac: _____
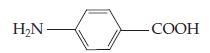
(c) PABA (formerly in sunscreen): ______
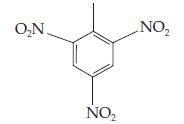
(d) TNT, trinitrotoluene: ______
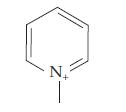
(e) N-Methylpyridinium: ______
HIN -CF3
Step by Step Solution
★★★★★
3.38 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a HCO3 the bicarbonate ion base HCO3 the bicarbonate ion is a base in the BrnstedLowry sense because ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


