Snapshots of two hypothetical reactions, A(g) +B(g) AB(g) and X(g) + Y(g) XY(g) at five
Question:
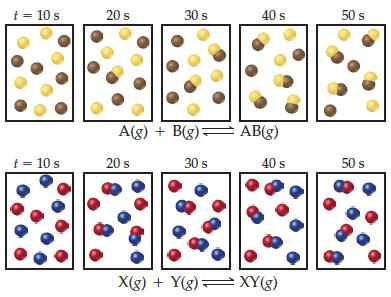
Snapshots of two hypothetical reactions, A(g) +B(g) ⇌ AB(g) and X(g) + Y(g) ⇌ XY(g) at five different times are shown here. Which reaction has a larger equilibrium constant?
Transcribed Image Text:
t = 10 s 20 s 30 s 40 s 50 s A(g) + B(g)= AB(g) t= 10 s 20 s 30 s 40 s 50 s X(g) + Y(g) : = XY(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
Equilibrium constant is mainly dependent on temperature of t...View the full answer

Answered By

Suneel Kumar
I completed my masters in Accountancy and finance and started doing job . I have a good enough interest and expertise in teachin g students. Exploring subject through a tutor will make me a good knowledge.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
1. A firm consists of 600 acres of land, of which 500 acres will be planted with corn, soybeans, and wheat according to this condition: -At least half of the planted acreage should be in corn - No...
-
24. A uniform chain of length L and mass M is lying on a smooth table and one third of its length is hanging vertically down over the edge of the table. If g is acceleration due to gravity, the work...
-
A culture of yeast that requires uracil for growth (urα3ÍË was mutagenized, and two mutant colonies, X and Y, have been isolated. Mating type a cells of mutant X arc mated...
-
The average of 4 consecutive odd positive integers is 16. The product of the smallest and largest positive odd integer is 1. 210 2. 247 3. 294 4. 320 None of the above 5.
-
Water flows steadily into a well-insulated electrical water heater (see Anim. 4- 1-1) with a mass flow rate of 1 kg/s at 100 kPa and 25C. Determine: The electrical power consumption if the water...
-
Consider the $1.90 per person per day as the relevant international poverty line and define an appropriate weekly poverty line. Compute the poverty severity index for this population of households....
-
Consider the soft drink delivery time data in Example 3.1. Example 3.1 a. Find the simple correlation between cases \(\left(x_{1} ight)\) an distance \(\left(x_{2} ight)\). b. Find the variance...
-
Beginning inventory, purchases, and sales for WCS12 are as follows: Assuming a perpetual inventory system and using the weighted average method, determine (a) The weighted average unit cost after the...
-
flower pot sitting on a table. Sign Convention: Force Diagram: Net Force Equation(s): , Subscript Definitions: A chandelier hanging from a chain. Sign Convention: Force Diagram: Net Force...
-
Your sister has just won $300,000 (taxfree) in the state lottery. Shes decided to quit her job and devote herself to writing novels for the next ten years, using her lottery winnings to support...
-
As shown in Figure 14.24, the first step in the heterogeneous hydrogenation of ethylene is adsorption of the ethylene molecule on a metal surface. One proposed explanation for the sticking of...
-
Suppose that, in the absence of a catalyst, a certain biochemical reaction occurs x times per second at normal body temperature (37C). In order to be physiologically useful, the reaction needs to...
-
The figure shows Lees utility of wealth curve. Lee is offered a job as a salesperson in which there is a 50 percent chance that she will make $4,000 a month and a 50 percent chance that she will make...
-
Which Organizational citizenship behavior or task performance more important, why?
-
Question 3. You participate in an Instagram contest and you are offered the following prizes. If the annual interest rate is 8% APR, which one is the most valuable prize? Show your calculations for...
-
For the Zero-Coupon tab, 1.Calculate the series of one-year forward rates for each year between 2006 - 2020, i.e., 2f1, 3f1, 4f1, 5f1, 6f1, 7f1, 8f1, 9f1, 10f1. 2.Plot the series of one-year forward...
-
To what extent are organizational citizenship behavior, organizational justice and psychological contract theories applicable to Chinese organizations and why? What is the suitable measure scales for...
-
How can leaders use motivation and organizational citizenship behavior to foster follower commitment to organizational goals?
-
Can portions of one loan secured by a residence consist of both acquisition indebtedness and home-equity indebtedness? Explain.
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
The first ionization energy and electron affinity of Ar are both positive values. (a) What is the significance of the positive value in each case? (b) What are the units of electron affinity?
-
If the electron affinity for an element is a negative number, does it mean that the anion of the element is more stable than the neutral atom? Explain.
-
Although the electron affinity of bromine is a negative quantity, it is positive for Kr. Use the electron configurations of the two elements to explain the difference.
-
Suppose you invest 52%, 28%, and 20% of your wealth into a stock, the market, and a risk-free asset, respectively. The beta of the stock is 1.1. What is the beta of the portfolio?
-
An investment of $21745, earning compound interest, grows by $2278 in one year. At this rate of growth, how long will it take for the original investment to double?
-
On September 12, 3,400 shares of Denver Company's common stock are acquired at a price of $58 per share plus a $170 brokerage commission. On October 15, an $1.20-per-share dividend was received on...

Study smarter with the SolutionInn App


