Succinic acid (H 2 C 4 H 6 O 4 ), which we will denote H 2
Question:
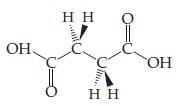
Succinic acid (H2C4H6O4), which we will denote H2Suc, is a biologically relevant diprotic acid with the structure shown below. It is closely related to tartaric acid and malic acid (Figure 16.1). At 25 °C, the acid-dissociation constants for succinic acid are Ka1 = 6.9 × 10-5 and Ka2 = 2.5 × 10-6.
(a) Determine the pH of a 0.32 M solution of H2Suc at 25 °C, assuming that only the first dissociation is relevant.
(b) Determine the molar concentration of Suc2- in the solution in part (a).
(c) Is the assumption you made in part (a) justified by the result from part (b)?
(d) Will a solution of the salt NaHSuc be acidic, neutral, or basic?
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





