Question: The radial probability function for a 2s orbital is shown here. Classify the following statements as either true or false: (a) There are two maxima
The radial probability function for a 2s orbital is shown here.
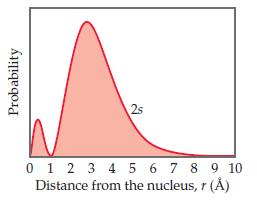
Classify the following statements as either true or false:
(a) There are two maxima in this function because one electron spends most of its time at an approximate distance of 0.5 Å from the nucleus and the other electron spends most of its time at an approximate distance of 3 Å from the nucleus.
(b) The radial probability function shown here and the probability density [Ψ(r)]2 both go to zero at the same distance from the nucleus, approximately 1 Å.
(c) For an s orbital, the number of radial nodes is equal to the principal quantum number, n.
Probability 2s 0 1 2 3 4 5 6 7 8 9 10 Distance from the nucleus, r ()
Step by Step Solution
3.27 Rating (162 Votes )
There are 3 Steps involved in it
a There are two maxima in this function because one electron spends most of its time at an approx... View full answer

Get step-by-step solutions from verified subject matter experts


