Use the solubility-product constant for Cr(OH) 3 (K sp = 6.7 10 -31 ) and the
Question:
Use the solubility-product constant for Cr(OH)3 (Ksp = 6.7 × 10-31) and the formation constant for Cr(OH)4 from Table 17.1 to determine the concentration of Cr(OH)4 in a solution that is buffered at pH = 10.0 and is in equilibrium with solid Cr(OH)3.
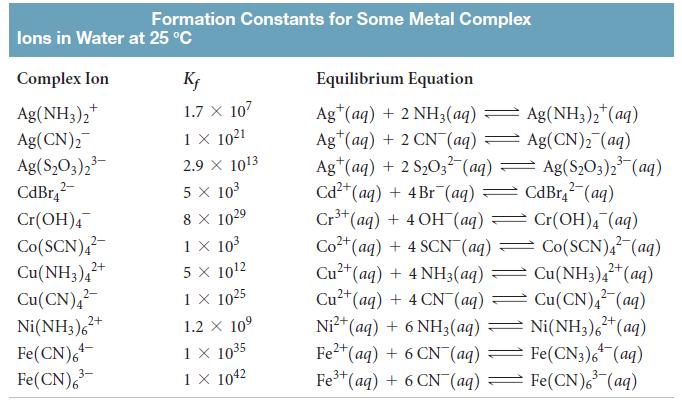
Transcribed Image Text:
Formation Constants for Some Metal Complex lons in Water at 25 °C Complex Ion K; Equilibrium Equation Ag(NH3),* Ag(CN)2 Ag(S,O3),3- CdBr,- Ag" (aq) + 2 NH3(aq) = Ag(NH3)2*(aq) Ag*(aq) + 2 CN (aq) Ag(CN)2 (aq) Ag*(ag) + 2 S203 (aq) = Ag(S,03)2 (aq) Cd2+(aq) + 4Br (aq) Cr*(aq) + 4OH (aq) Cr(OH), (aq) Co2+(aq) + 4 SCN (aq) Cu2+(aq) + 4 NH3(aq) Cu2+(aq) + 4 CN (aq) = 2+(aq)+6 NH3(aq) 1.7 X 107 Ag(NH3),*(aq) 1 × 1021 2.9 x 1013 Ag(S,O3)2 (aq) 5 x 103 CdBr, (aq) Cr(OH)4 8 X 1029 Co(SCN),- Cu(NH3),+ Cu(CN), Ni(NH3),+ Fe(CN),- Fe(CN), 1 x 103 5 x 1012 1X 1025 Co(SCN), (aq) Cu(NH3),*(aq) Cu(CN), (aq) 2+ 2- Ni²* (aq) + 6 NH3(aq) = Ni(NH3),*(aq) Fe"(aq) + 6 CN (aq) = Fe(CN3), (aq) Fe (aq) + 6 CN (aq) 1.2 X 10° 1 X 1035 1 X 1042 4- 3- = Fe(CN), (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
The formation of complex ion CrOH4 involves two reaction steps CrOH3s Cr 3 aq ...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Calculate the solubility-product constant for each of the following substances, given that the molar concentrations of their saturated solutions are as indicated: (a) RaSO4 (6.6 x 10-6 M). (b)...
-
The solubility-product constant for Ce(IO3)3 is 3.2 x 10-10. What is the Ce3+ concentration in a solution prepared by mixing 50.00 mL of 0.0450 M Ce3+ with 50.00 mL of? (a) Water? (b) 0.0450 M IO3-?...
-
The solubility-product constant for barium permanganate, Ba(MnO 4 ) 2 , is 2.5 10 -10 . Assume that solid Ba(MnO 4 ) 2 is in equilibrium with a solution of KMnO 4 .What concentration of KMnO 4 is...
-
Linking every transport stakeholder together and ensuring seamless travel across Europe is a dream. With this objective, Amadeus, a leading global travel technology player, initiated a novel idea of...
-
What is wrong with this statement of purpose? PURPOSE: Determine if it takes too long to get cash from the automated teller machine during the lunch hour. Give an improved statement of purpose.
-
Do beavers benefit beetles? Researchers laid out 23 circular plots, each 4 meters in diameter, at random in an area where beavers were cutting down cottonwood trees. In each plot, they counted the...
-
Consider the model \[ y=\theta_{1}-\theta_{2} e^{-\theta_{3} x}+\varepsilon \] This is called the Mitcherlich equation, and it is often used in chemical engineering. For example, \(y\) may be yield...
-
1. Should Cheerios have been more sensitive to cultural issues? 2. Do you think this ad will increase sales of Cheerios? The case describes the issues surrounding a commercial for Cheerios which...
-
An electricity company pays a dividend $ 1 . 6 4 per share and the share price is $ 2 7 . a ) If the investors believe that the dividend growth rate will be 3 % annually, what will be the yield that...
-
1. What types of organizational changes would you advise Japanese electronics managers to consider? 2. How do you think Japanese demographic trends have been a factor in the innovation problem? Japan...
-
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca 5 (PO 4 ) 3 OH, and whose corresponding Ksp = 6.8 10 -27 .As discussed in the Chemistry and Life box on page 730, fluoride in...
-
Calculate the solubility of Mg(OH) 2 in 0.50 M NH 4 Cl.
-
Discuss how the presence of a strong labor union may change the nature of the market in which rank-and-file employees negotiate with their employer.
-
Can the original balanced scorecard by Kaplan and Norton be applied in a supply chain setting? Why or why not?
-
Explain which cost items cannot be easily summed up when measuring the performance of a whole supply chain (macro level).
-
Explain the concept of Value-at-risk (VaR) as a key risk measure. How is VaR typically calculated?
-
Explain the conditions for employing ABC in a supply chain network.
-
Why do an individual firms attempts to improve its C2C cycle not always lead to a corresponding improvement for the entire supply chain?
-
Blue Corporation, a manufacturing company, decided to develop a new line of merchandise. The project began in 2015. Blue had the following expenses in connection with the project: The new product...
-
Inexhaustible collections of ONPOs are not required to be capitalized or depreciated, if certain criteria are met. Why is this so, and what accounting and reporting recognition, if any, is required...
-
A 50-lossless line of length l = 0 375connects a 300-MHz generator with Vg = 300 V and Zg = 50 to a load ZL. Determine the time-domain current through the load for: (a) ZL =...
-
A generator with Vg = 300 V and Zg = 50 is connected to a load ZL = 75 through a 50-lossless line of length l = 0 15. (a) Compute Zin, the input impedance of the line at the...
-
If the two-antenna configuration shown in Fig. 2-41 (P2.32) is connected to a generator with Vg =250 V and Zg = 50 , how much average power is delivered to each antenna?
-
Company name is Walmart, Inc. here is the link https://www.sec.gov/ix?doc=/Archives/edgar/data/104169/000010416923000020/wmt-20230131.htm Overview 1. The financial statements for your company are...
-
Congress would like to increase tax revenues by 19 percent. Assume that the average taxpayer in the United States earns $54,000 and pays an average tax rate of 15 percent. Required: a. If the income...
-
Best Solutions is a retail merchandiser selling computer equipment. Best uses the gross method of accounting for inventory purchases and sales, a perpetual inventory system with LIFO inventory...

Study smarter with the SolutionInn App


