Which of the following statements about the bonding atomic radii in Figure 7.7 is incorrect? (i) For
Question:
Which of the following statements about the bonding atomic radii in Figure 7.7 is incorrect?
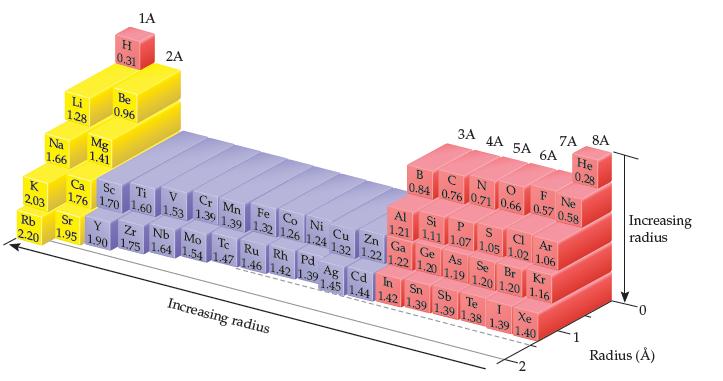
(i) For a given period, the radii of the representative elements generally decrease from left to right across period.
(ii) The radii of the representative elements for the n = 3 period are all larger than those of the corresponding elements in the n = 2 period.
(iii) For most of the representative elements, the change in radius from the n = 2 to the n = 3 period is greater than the change in radius from n = 3 to n = 4.
(iv) The radii of the transition elements generally increase moving from left to right within a period.
(v) The large radii of the Group 1A elements are due to their relatively small effective nuclear charges.
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





