Calculate the binding energy per nucleon of 4 He, the particle. Strategy To find BE/A, we
Question:
Calculate the binding energy per nucleon of 4He, the α particle.
Strategy
To find BE/A, we first find BE using the Equation ![]() and then divide by A. This is straightforward once we have looked up the appropriate atomic masses in Appendix A.
and then divide by A. This is straightforward once we have looked up the appropriate atomic masses in Appendix A.
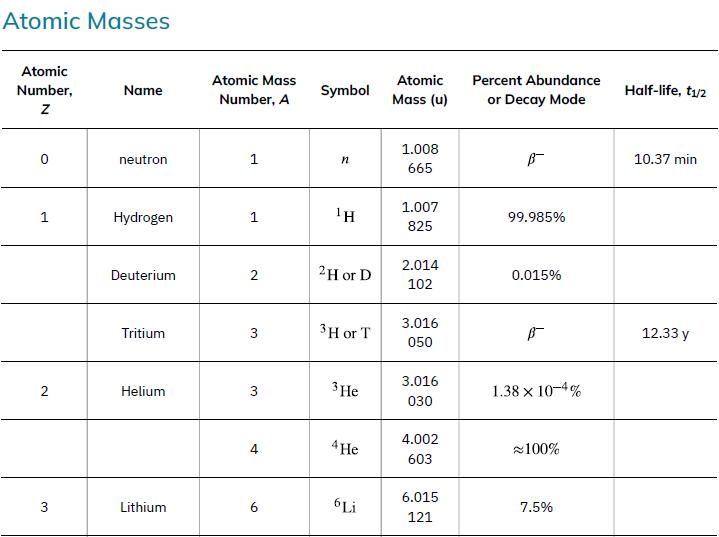
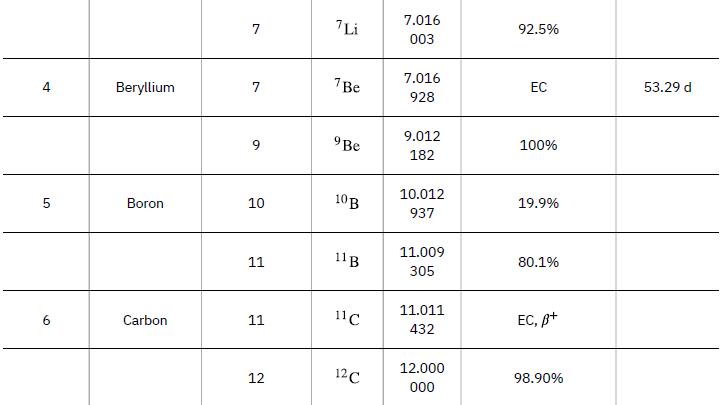
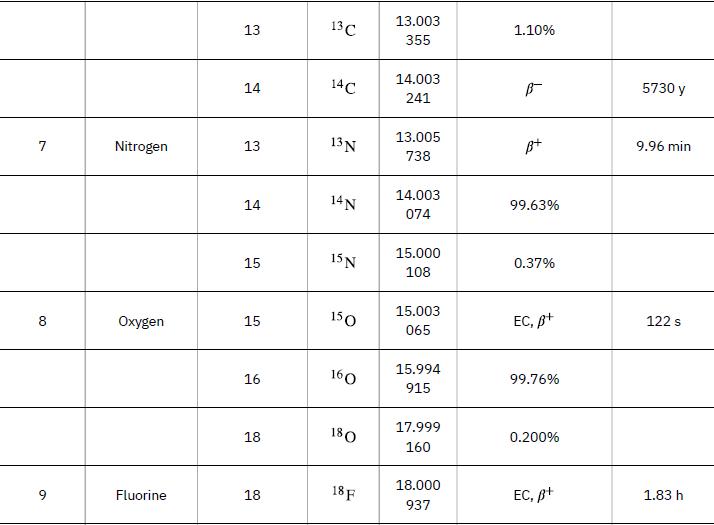
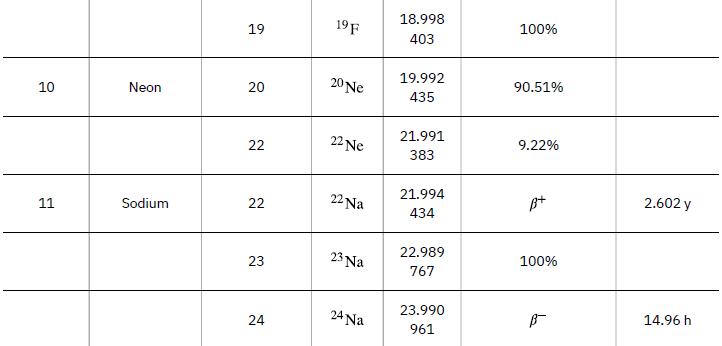
Data from Appendix A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





