Question: A 2.0 eV photon is incident on an atom in the (p) stat shown in the energy-level diagram in Figure Q29.22. Does the atom undergo
A 2.0 eV photon is incident on an atom in the \(p\) stat shown in the energy-level diagram in Figure Q29.22. Does the atom undergo an absorption transition, a stimulated emission transition, or neither? Explain.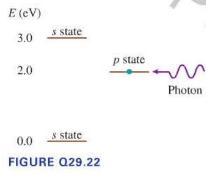
E (eV) 3.0 2.0 s state p state N 0.0 & state FIGURE Q29.22 Photon
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
The atom undergoes neither an absorption transition nor a stimulated emission transition In an a... View full answer

Get step-by-step solutions from verified subject matter experts


