Figure Q29.20 shows the energy (quad E(mathrm{eV})) levels of a hypothetical atom. (quad(n+1) p) a. What minimum
Question:
Figure Q29.20 shows the energy \(\quad E(\mathrm{eV})\) levels of a hypothetical atom. \(\quad(n+1) p\)
a. What minimum kinetic energy (in \(\mathrm{eV}\) ) must an electron have to collisionally excite this atom and cause the emission of a \(620 \mathrm{~nm}\) photon? Explain.
b. Can an electron with \(K=6 \mathrm{eV}\) cause the emission of \(620 \mathrm{~nm}\) light? If so, what is the final kinetic energy of the
c. Can a \(6 \mathrm{eV}\) photon cause the emission of \(620 \mathrm{~nm}\) light from this atom? Why or why not?
d. Can a \(7 \mathrm{eV}\) photon cause the emission of \(620 \mathrm{~nm}\) light from this atom? Why or why not?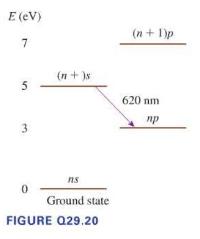
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:





