Question: Consider the process described by Figure P16.21. If 500 J of heat are added to the system during the second step, what is the change
Consider the process described by Figure P16.21. If 500 J of heat are added to the system during the second step, what is the change in the internal energy of the system during this step?
Figure 16.21
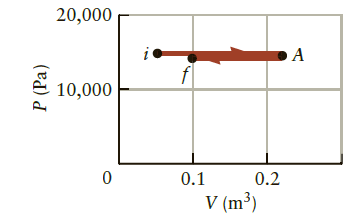
20,000 10,000 0.1 0.2 V (m) P (Pa)
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
The first law of thermodynamics relates the change of internal energy to the heat ... View full answer

Get step-by-step solutions from verified subject matter experts


