(a) Show that the number of electron states in a sub-shell is 4 + 2. (b) By...
Question:
(a) Show that the number of electron states in a sub-shell is 4ℓ + 2.
(b) By summing the number of states in each of subshells, show that the number of states in a shell is 2n2. Use the fact that the sum of the first n odd integers, from 1 to 2n − 1, is n2. That comes from regrouping the sum in pairs, starting by adding the largest to the smallest:
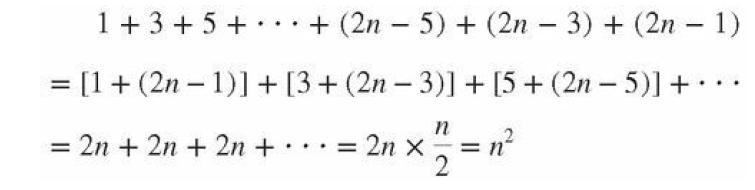
Transcribed Image Text:
1+ 3 + 5 + + (2n-5) + (2n - 3) + (2n-1) = [1 + (2n-1)] + [3+ (2n-3)] + [5+ (2n-5)] + .. = 2n + 2n + 2n + = nx²2/² = n² ... X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a A subshell is a collection of orbitals with the same n and values The number o...View the full answer

Answered By

Tamondong Riza
Professionally, I am a teacher with years of experience tutoring math and science, as well as teaching in both public schools and independent schools. I feel that education should be an enlightening experience for all children, and I'm committed to helping my students learn new skills and make progress in their subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:
Students also viewed these Sciences questions
-
(a) Show that the number of electron states in a subshell is 4 + 2. (b) By summing the number of states in each of the subshells, show that the number of states in a shell is 2n2. The sum of the...
-
Show that the number of different electron states possible for a given value of n is 2n2.
-
The controller for Beckham Company believes that the number of direct labor hours is associated with overhead cost. He collected the following data on the number of direct labor hours and associated...
-
State DMV records indicate that of all vehicles undergoing emissions testing during the previous year, 70% passed on the first try. A random sample of 200 cars tested in a particular county during...
-
In a test of the effects of lifting heavy weights on blood pressure, one group undergoes a treatment consisting of a weight-lifting program while another group lifts tennis balls. Identify any...
-
York Steel Corporation produces iron rings that are supplied to other companies. These rings are supposed to have a diameter of 24 inches. The machine that makes these rings does not produce each...
-
What is products liability? Describe what legal theories an injured party can pursue when filing a lawsuit against a seller, manufacturer, or supplier of goods. Describe the defenses often used in...
-
Idaho Food Processors processes potatoes into french fries. Production requires two processes: cutting and cooking. Direct materials are added at the beginning of the cutting process (potatoes) and...
-
As you know, the value of. It is defined as the ratio of the circumference of a circle C divided by its diameter 2r. That is x-C/2. a) Let's assume that you measured a circumference of a circle to be...
-
Mill Corporation acquired 100 percent ownership of Roller Company on January 1, 20X8, for $128,000. At that date, the fair value of Rollers buildings and equipment was $20,000 more than the book...
-
The neutrons produced in fission reactors have a wide range of kinetic energies. After the neutrons make several collisions with atoms, they give up their excess kinetic energy and are left with the...
-
A particle is confined to & finite box of length L. In the nth state, the wave function has n 1 nodes. The wave function must make a smooth transition from sinusoidal inside the box to a decaying...
-
Bradley has two college-age children, Clint, a freshman at State University, and Abigail, a junior at Northwest University. Both Clint and Abigail are full-time students. Clint's expenses during the...
-
Does inclusive leadership, transformational leadership, or leader-member exchange contribute most to employee engagement, innovation, and productivity across Western and Eastern cultures? Explain....
-
Brooke the operator of a restaurant served a dinner to Adam. Adam had nearly finished his soup when he found several metal chips in the bottom of her bowl. in this case with the regard to the dinner...
-
Jack (age 54) and Reese (52) Holmes are a married couple with two children, Mattie (24) and Rose (30). Rose has 2 kids, Macie and Wade (ages 2 and 4). Jack Holmes is an oral surgeon, and he co-owns a...
-
Choose the best definition of residual risk. O Residual risk is the risk to a business process of a cybersecurity leak. O Residual risk is the risk to a business process of a natural disaster, such...
-
The stockholders equity T accounts of I-Cards Inc. for the fiscal year ended December 31, 2016, are as follows. Common Stock Jan. 1 Balance 5,265,000 Apr. 14 Issued 33,000 shares 1,287,000 Dec. 31...
-
A parabola with an equation in the form y = ax2 + bx + c passes through the points (-2, -32), (1, 7), and (3, 63). a. Set up systems and use matrices to find the values of a, b, and c for this...
-
Vince, Inc. has developed and patented a new laser disc reading device that will be marketed internationally. Which of the following factors should Vince consider in pricing the device? I. Quality of...
-
Predict the value for the specific rotation of the following compound. Explain your answer. - , .
-
Identify whether each of the following compounds exhibits a molecular dipole moment. For compounds that do, indicate the direction of the net molecular dipole moment: a. CHCl 3 b. CH 3 OCH 3 c. NH 3...
-
Which of the following compounds has the larger dipole moment? Explain your choice: CHCl 3 or CBrCl 3
-
Samsung is a leading global manufacturer that competes with Apple and Google. Key financial figures for Samsung, Apple, and Google follow. $ millions Average assets Net income Revenues Required:...
-
In each case below, determine if the taxpayer has received income: Al was sent on assignment to work at a temporary location. Al received a check in early January which he could have picked-up on...
-
In January 2 0 1 7 , Saint Peter Pharmacy bought machinery for $ 4 8 , 0 0 0 . It was decided that this machinery should be depreciated using the straight - line method over a period of 1 0 years...

Study smarter with the SolutionInn App


