Hydrogen bonding is responsible for many of the unusual properties of water (see Sec. 16.1). A simplified
Question:
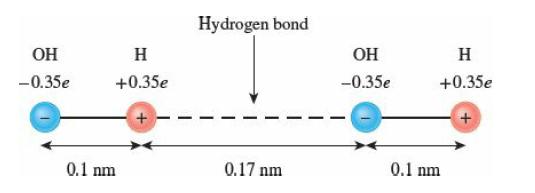
Hydrogen bonding is responsible for many of the unusual properties of water (see Sec. 16.1). A simplified model represents a hydrogen bond as the electrostatic interaction of four point charges arranged along a straight line, as shown in the figure.
(a) Using this model, estimate the energy that must be supplied to break a single hydrogen bond.
(b) Estimate the energy that must be supplied to break the hydrogen bonds in 1 kg of liquid water and compare it with the heat of vaporization of water. Assume that the number of hydrogen bonds is equal to the number of molecules. Is it coincidence that these two quantities are similar? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:





