Question: You are to simulate a partial condenser module that converts a vapor feed stream (SF) containing a single condensable species at temperature TF(K) to liquid
You are to simulate a partial condenser module that converts a vapor feed stream (SF) containing a single condensable species at temperature TF(K) to liquid and vapor product streams (SL, SV) in equilibrium at a temperature (TK). The process takes place at a constant pressure (Patm). The compositions of the liquid and vapor product streams are related by Raoult’s law (Equation 6.4-1), and the component vapor pressures are correlated with temperature by the Antoine equation, Table B.4.
The system variables are as follows:

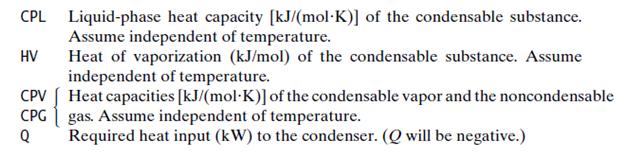
Show that the system has five degrees of freedom, counting as the system variables three stream flow rates, two mole fractions, one vapor pressure TF, T, P, and Q.
(b) The input variables to the module are to be the molar flow rates of the condensable and non condensable feed stream constituents [SF (1) and SF (2)], the feed temperature [SF (3) = TF], the operating pressure of the condenser (P), and the fraction YC of the condensable species in the feed that is to be condensed. (The heat capacities and heat of vaporization must also be supplied.) The output variables are the molar flow rate of the liquid product stream [SL(1)], the molar flow rates of the vapor product stream constituents [SV(1) and SV(2)], the operating temperature of the condenser [SL (2) = SV(3) = T], and the required heat transfer rate (Q). Outline the required calculations.
(c) Write a spreadsheet to perform the calculations of part (b) for a feed stream flowing at 1.00 molls containing methyl alcohol in air at 60°C and 1 atm with a relative saturation of 85%, from which 90% of the methanol is to be removed by partial condensation at constant pressure. The heat capacities in kli(mol•K) of liquid methanol, methanol vapor, and air may be taken to be 0.078, 0.050, and 0.030, respectively. After you have generated the solution and recorded the output variable values, use the spreadsheet to generate a plot of condenser temperature (T) versus fractional methanol removal (YC), and briefly explain why the plot looks the way it does.
(d) Use an equation-solving program to perform the calculations outlined in part (c).
(e) Write a computer subprogram CNDNS to implement the procedure of part (b) for the test case of part (c). The subroutine arguments should be SF, SV, SL, P, YC, and Q. Input variables are YC, P, and the attributes of SF, and output variables are Q and the attributes of SV and SL. The values of physical property parameters, A, B, C, CPL, CPV, CPG, and HV should be transmitted to the subprogram either as additional arguments or through a COMMON or GLOBAL statement. Then write and run a calling program that defines the attributes of SF and other input and physical property parameters, calls the subprogram, and prints out the input and output variable values for the test case of part (c).
NF, NL, NV mol/s of feed, liquid product, and vapor product. XF Mole fractions of the condensable substance in the feed and the vapor product. Feed temperature (K), condenser temperature (K), and condenser pressure (atm), respectively. Vapor pressure (mm Hg) of the condensable substance at temperature T. Antoine equation constants for the condensable substance. XV TF,T,P PV , ,
Step by Step Solution
3.24 Rating (159 Votes )
There are 3 Steps involved in it
a The system has five degrees of freedom counting as the system variables three stream flow rates NF ... View full answer

Get step-by-step solutions from verified subject matter experts


