Question: We have this data from an experiment in which NaOH reacts with an unknown acid. Trial 1 2 3 4 5 6 7 8
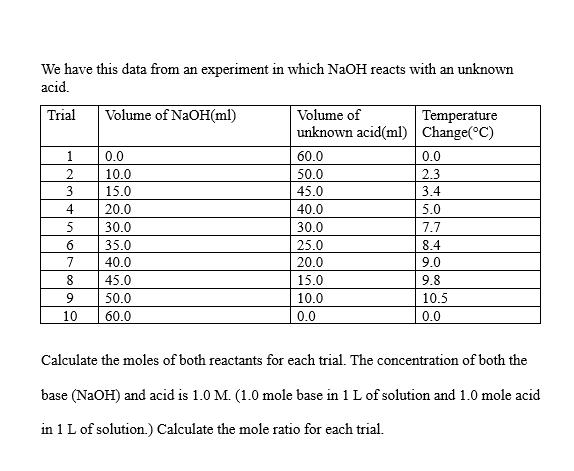
We have this data from an experiment in which NaOH reacts with an unknown acid. Trial 1 2 3 4 5 6 7 8 9 10 Volume of NaOH(ml) 0.0 10.0 15.0 20.0 30.0 35.0 40.0 45.0 50.0 60.0 Volume of unknown acid(ml) 60.0 50.0 45.0 40.0 30.0 25.0 20.0 15.0 10.0 0.0 Temperature Change(C) 0.0 2.3 3.4 5.0 7.7 8.4 9.0 9.8 10.5 0.0 Calculate the moles of both reactants for each trial. The concentration of both the base (NaOH) and acid is 1.0 M. (1.0 mole base in 1 L of solution and 1.0 mole acid in 1 L of solution.) Calculate the mole ratio for each trial.
Step by Step Solution
3.54 Rating (161 Votes )
There are 3 Steps involved in it
Trial Volume of NaOHml Volume of unknown acidml Temperature ChangeC Moles of NaOH Moles of unknown a... View full answer

Get step-by-step solutions from verified subject matter experts


