An expression for the value of (c_{p}) for carbon dioxide as a function of temperature is [
Question:
An expression for the value of \(c_{p}\) for carbon dioxide as a function of temperature is
\[ c_{p}=286-\frac{1.15 \times 10^{5}}{T}+\frac{2.49 \times 10^{6}}{T^{2}} \]
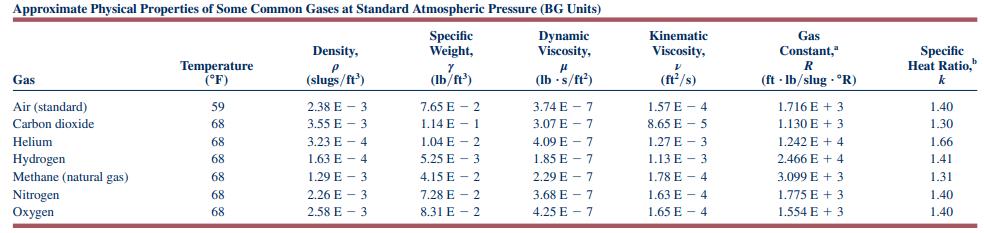
where \(c_{p}\) is in \((\mathrm{ft} \cdot \mathrm{lb}) /\left(\mathrm{lbm} \cdot{ }^{\circ} \mathrm{R}\right)\) and \(T\) is in \({ }^{\circ} \mathrm{R}\). Compare the change in enthalpy of carbon dioxide using the constant value of \(c_{p}\) (see Table 1.7) with the change in enthalpy of carbon dioxide using the expression above, for \(T_{2}-T_{1}\) equal to
(a) \(10^{\circ} \mathrm{R}\),
(b) \(1000{ }^{\circ} \mathrm{R}\),
(c) \(3000{ }^{\circ} \mathrm{R}\). Set \(T_{1}=540{ }^{\circ} \mathrm{R}\).
Table 1.7
Step by Step Answer:

Munson Young And Okiishi's Fundamentals Of Fluid Mechanics
ISBN: 9781119080701
8th Edition
Authors: Philip M. Gerhart, Andrew L. Gerhart, John I. Hochstein





