Question: A flat surface containing many parallel pores is clogged with coke from a manufacturing process, as shown in the figure below. Pure oxygen gas (O
a. At some time after the oxidation process, the cleaned depth of the pore is 3.0 mm (0.3 cm) from the mouth of the pore. What is the total emissions rate of CO2 gas (WB) at this point in the process?
b. How long will the oxidation process take to reach this cleaned depth of 0.3 cm from the mouth of the pore?
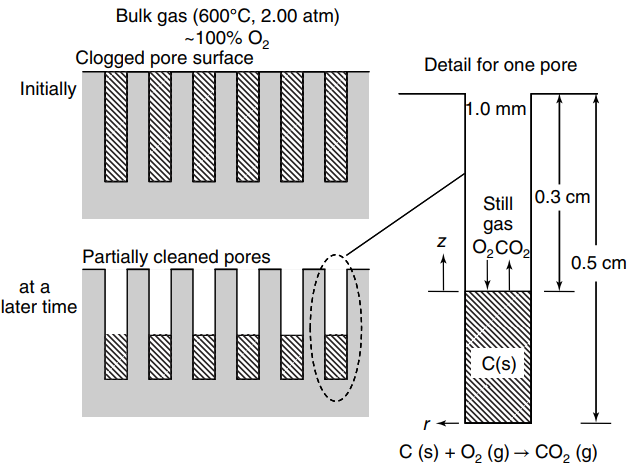
Bulk gas (600C, 2.00 atm) -100% O2 Clogged pore surface Detail for one pore Initially 1.0 mm Still 0.3 cm gas 0,CO2 Partially cleaned pores 0.5 cm at a later time C(s) C (s) + O2 (g) CO, (g)
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Assume 1 PSS system 2 No homogeneous reaction 3 1D flux along z EMCD a Determine W B ... View full answer

Get step-by-step solutions from verified subject matter experts


