Question: Calculate E 0 for the process Cu(NH3)42+ + Cu(NH3)2 + 2 NH; given that Cut + 2NH3Cu(NH3)2 B2 = 7.2 X 10 2+ Cu+ +
Calculate E0 for the process
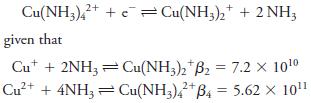
Cu(NH3)42+ + Cu(NH3)2 + 2 NH; given that Cut + 2NH3Cu(NH3)2 B2 = 7.2 X 10 2+ Cu+ + 4NH3 = Cu(NH3)42+ B4 = 5.62 10
Step by Step Solution
★★★★★
3.48 Rating (165 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

E0 for the process CuNH3 2 CuNH 3 2 2 NH3 can be calculated using the standard red... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


