One common way to determine phosphorus in urine is to treat the sample after removing the protein
Question:
One common way to determine phosphorus in urine is to treat the sample after removing the protein with molybdenum (VI) and then reducing the resulting 12-molybdophosphate complex with ascorbic acid to give an intense blue-colored species called molybdenum blue. The absorbance of molybdenum blue can be measured at 650 nm. A 24-hour urine sample was collected, and the patient produced 1122 mL in 24 hours. A 1.00-mL aliquot of the sample was treated with Mo(VI) and ascorbic acid and diluted to a volume of 50.00 mL. A calibration curve was prepared by treating 1.00 mL aliquots of phosphate standard solutions in the same manner as the urine sample. The absorbances of the standards and the urine sample were obtained at 650 nm and the following results obtained:
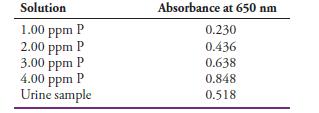
(a) Find the slope, intercept, and standard error in y of the calibration curve. Construct a calibration curve. Determine the concentration number of phosphorus in ppm in the urine sample and its standard deviation from the least-squares equation of the line. Compare the unknown concentration to that obtained manually from a calibration curve.
(b) What mass in grams of phosphorus was eliminated per day by the patient?
(c) What is the phosphate concentration in urine in mM?
Step by Step Answer:

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch





