Water can be determined in solid samples by infrared spectroscopy. The water content of calcium sulfate hydrates
Question:
Water can be determined in solid samples by infrared spectroscopy. The water content of calcium sulfate hydrates is to be measured using calcium carbonate as an internal standard to compensate for some systematic errors in the procedure. A series of standard solutions containing calcium sulfate dihydrate and a constant known amount of the internal standard is prepared. The solution of unknown water content is also prepared with the same amount of internal standard. The absorbance of the dihydrate is measured at one wavelength (Asample) along with that of the internal standard at another wavelength (Astd). The following results were obtained.
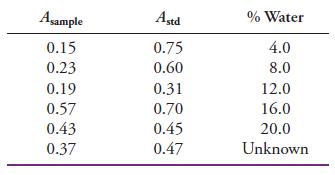
(a) Plot the absorbance of the sample (Asample) versus the % water, and determine whether the plot is linear from the regression statistics.
(b) Plot the ratio Asample/Astd versus % water, and comment on whether using the internal standard improves the linearity from that in part (a). If it improves the linearity, why?
(c) Calculate % water in the unknown using the internal standard data.
Step by Step Answer:

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch




