Suppose a new study is planned, with 200 patients randomly assigned to each of a 400 IU
Question:
Ophthalmology
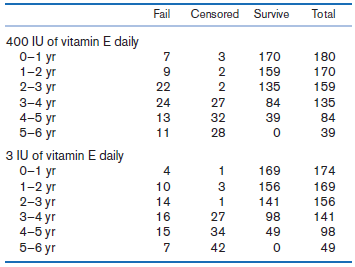
In Table 14.33, we present data from the RP clinical trial described in Example 14.30 (on page 813) concerning effect of high-dose vitamin E (400 IU/day) vs. low-dose vitamin E (3 IU/day) on survival (where failure is loss of at least 50% of initial ERG 30 Hz amplitude).
Table 14.33 Number of patients who failed, were censored, or survived by year in the 400 IU vitamin E group and 3 IU vitamin E group, respectively, RP clinical trial
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





