A stream of liquid nitrogen enters an adiabatic, steady-state valve as a saturated liquid at P =
Question:
A stream of liquid nitrogen enters an adiabatic, steady-state valve as a saturated liquid at P = 2 MPa. The material leaves the valve at P = 0.6 MPa. Use the data in Figure 2-3 to determine the following.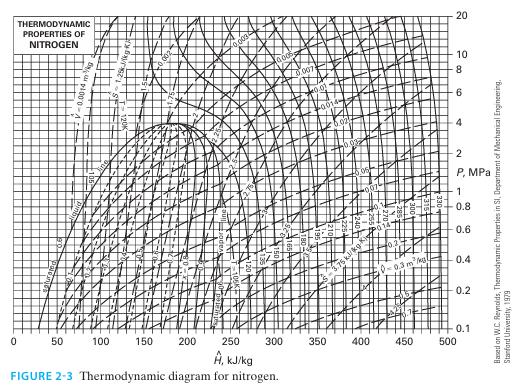 A. The temperature of the nitrogen leaving the valve.
A. The temperature of the nitrogen leaving the valve.
B. The physical state of the nitrogen leaving the valve (if VLE mixture, indicate quality).
C. The rate at which entropy is generated in the valve (per kg of entering nitrogen.)
D. The irreversible feature of the process, if any, that explains the result of part C.
E. Repeat parts A, B, and C for methane instead of nitrogen. Use Figure 7-1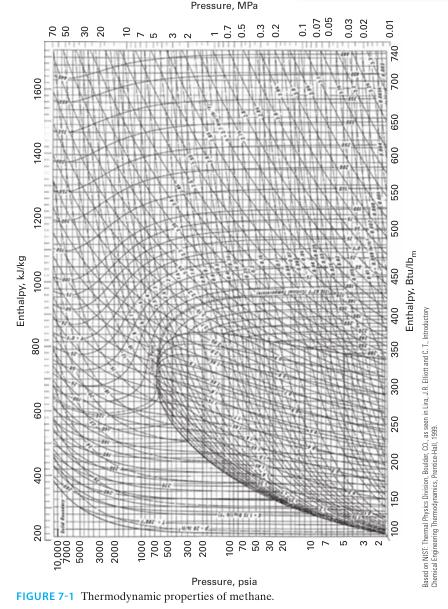
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





