Consider the P-T diagram in Figure 2-15 for a pure substance: A. What phase is represented by
Question:
Consider the P-T diagram in Figure 2-15 for a pure substance: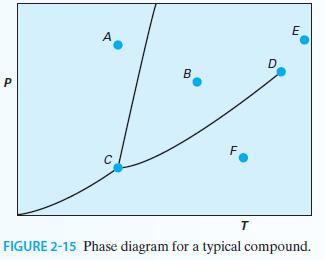 A. What phase is represented by point A? According to the Gibbs phase rule, how many degrees of freedom exist at this point?
A. What phase is represented by point A? According to the Gibbs phase rule, how many degrees of freedom exist at this point?
B. What phase is point B? How many degrees of freedom exist at this point?
C. How many phases are in equilibrium at point C? What is the name of this point? How many degrees of freedom exist at this point?
D. How many phases are in equilibrium at point D? What is the name of this point? How many degrees of freedom exist at this point?
E. What phase is point E?
F. What phase is point F?
G. What is the physical significance of the curve that exists between points C and D?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





