Ethyl acetate is synthesized from ethanol and acetic acid by the liquid phase esterification reaction shown in
Question:
Ethyl acetate is synthesized from ethanol and acetic acid by the liquid phase esterification reaction shown in Figure 15-3..
C2 H5 OH + CH3 COOH ↔ H2 O + CH3 COOC2 H5
Ethanol + acetic acid ↔ water + ethyl acetate
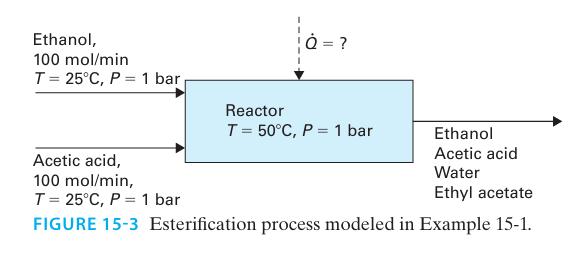
Two separate feeds containing 100 mol/min each of ethanol and acetic acid enter a steady-state reactor at T = 25°C and P = 1 bar. The reactor is maintained at P = 1 bar and is isothermal at T = 50°C, and large enough that the reaction can be modeled as progressing to equilibrium. Assume the liquid phase can be modeled as an ideal solution.
A. Determine the composition of the stream leaving the reactor.
B. Determine the rate at which heat must be added to or removed from the reactor in order to maintain a constant temperature of T = 50°C.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





