Ethyl acetate can be formed from ethanol and acetic acid, by the liquid phase reaction A liquid
Question:
Ethyl acetate can be formed from ethanol and acetic acid, by the liquid phase reaction
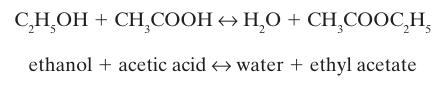
A liquid phase reactor (Figure 14-6) with a constant temperature T = 298.15 K initially contains 5 moles each of ethanol, acetic acid, water, and ethyl acetate. Find the contents of the reaction at equilibrium if
A. The reaction is carried out at constant P = 1 bar
B. The reaction is carried out at constant P = 50 bar
For the purposes of this problem, assume the mixture can be modeled as an ideal solution 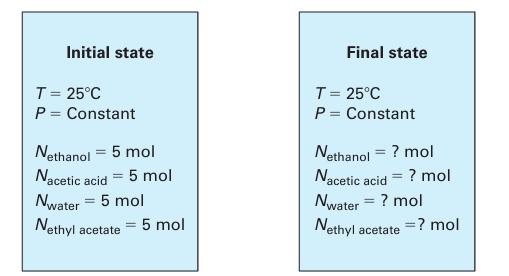
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





