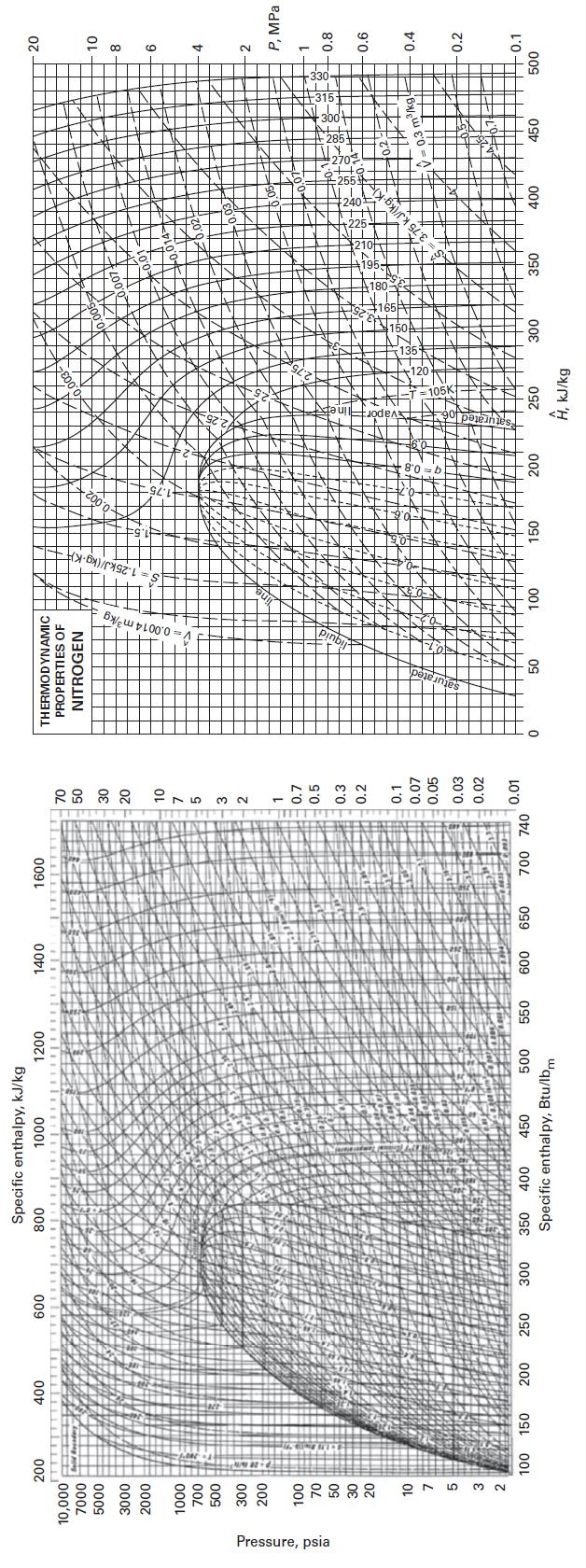
Find the molar volume of nitrogen at P = 10 bar and T = 330 K, using
Question:
Find the molar volume of nitrogen at P = 10 bar and T = 330 K, using the following methods.
A. The Soave equation of state
B. The Peng-Robinson equation of state
C. The virial equation of state
D. The Lee-Kesler generalized correlation
E. Appendix F
Appendix F
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





