For a mixture of methyl ethyl ketone (1) + water (2) at 45C, what would be the
Question:
For a mixture of methyl ethyl ketone (1) + water (2) at 45°C, what would be the predicted bubble-point pressure using Raoult’s Law if your mixture was 20% water by mole? Would you expect Raoult’s Law to be a good model for this system? Why or why not?.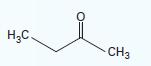
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





