Predict the Pxy behavior for a mixture of diethyl ether (1) + methanol (2) at 303.15 K
Question:
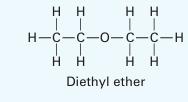
Predict the Pxy behavior for a mixture of diethyl ether (1) + methanol (2) at 303.15 K using the Peng Robinson equation of state. Compare the predictions to the experimental data given in Table P12-24. Using the OBJ_P objective function, calculate an optimal binary interaction parameter. Is the Peng-Robinson equation of state a reasonable model for this system at this state? Please explain.
![TABLE P12-24 Vapor-liquid equilibrium of diethyl ether (1) + methanol (2) at 303.15K. P[kPa] 25.035 39.243](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/4/0/1/115653b8b5ba33ee1698401110623.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





