Predict the Pxy behavior for a mixture of acetone (1) + 2-propanol (2) at 328.15 K using
Question:
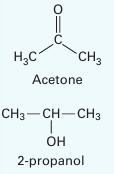
Predict the Pxy behavior for a mixture of acetone (1) + 2-propanol (2) at 328.15 K using the Peng-Robinson equation of state. Compare the predictions to the experimental data given in Table P12-26. Determine a “best fit” bi nary interaction parameter that best matches the equation of state pressures to the experimental pressures. Plot those new predictions on the same curve. Comment on your results.
![TABLE P12-26 Vapor-liquid equilib- rium of acetone (1) + 2-propanol (2) at 328.15K. P[kPa] 34.393 39.930](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/4/0/1/470653b8cbeb6e511698401465347.jpg)
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:




