Propylene (CH 3 2CH 5 CH 2 ) can be formed by the gas phase thermal cracking
Question:
Propylene (CH3 2CH5CH2 ) can be formed by the gas phase thermal cracking of n-butane:
![]()
A steady-state reactor (Figure 14-5) is maintained at a constant T = 500 K and a constant P. The feed to the reactor is 10 mol/s of n-butane. Assuming the stream leaving the reactor is at equilibrium, find the flow rate of propylene in the stream for each of the following cases
A. The vessel has P = 1 bar.
B. The vessel has P = 25 bar, and the contents are modeled as an ideal gas mixture.
C. The vessel has P = 25 bar, and the mixture fugacity of each compound is determined from the Peng-Robinson equation.
The reaction at 500 K has an equilibrium constant K500 = 0.931.
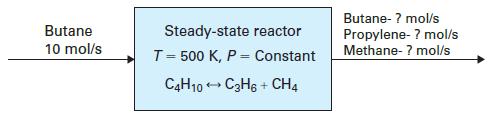
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





