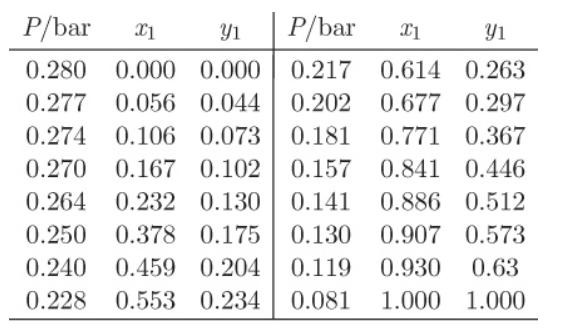
The data below are for the system 1,4 dioxane (1)/methanol(2) at 308.5 K. a) What is the
Question:
The data below are for the system 1,4 dioxane (1)/methanol(2) at 308.5 K.
a) What is the saturation pressure of methanol at 308.5 K?
b) What is the phase of a mixture that contains 60% dioxane at 308.5 K, 0.1 bar?
c) Determine the bubble and dew pressures of a mixture that contains 60% dioxane at 308.5 K.
These questions can be answered more easily if you plot the Pxy graph based on these data.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:





